Recycling method for valuable metal from waste nickel-cobalt-manganese lithium ion battery
A nickel-cobalt-manganese-lithium, ion battery technology is applied in the wet recycling of lithium valuable metals, cobalt, manganese, and nickel in waste nickel-cobalt-manganese lithium-ion batteries, and can solve the problems of high energy consumption and complicated operation, etc. To achieve the effect of simple equipment, simple operation and industrialized production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
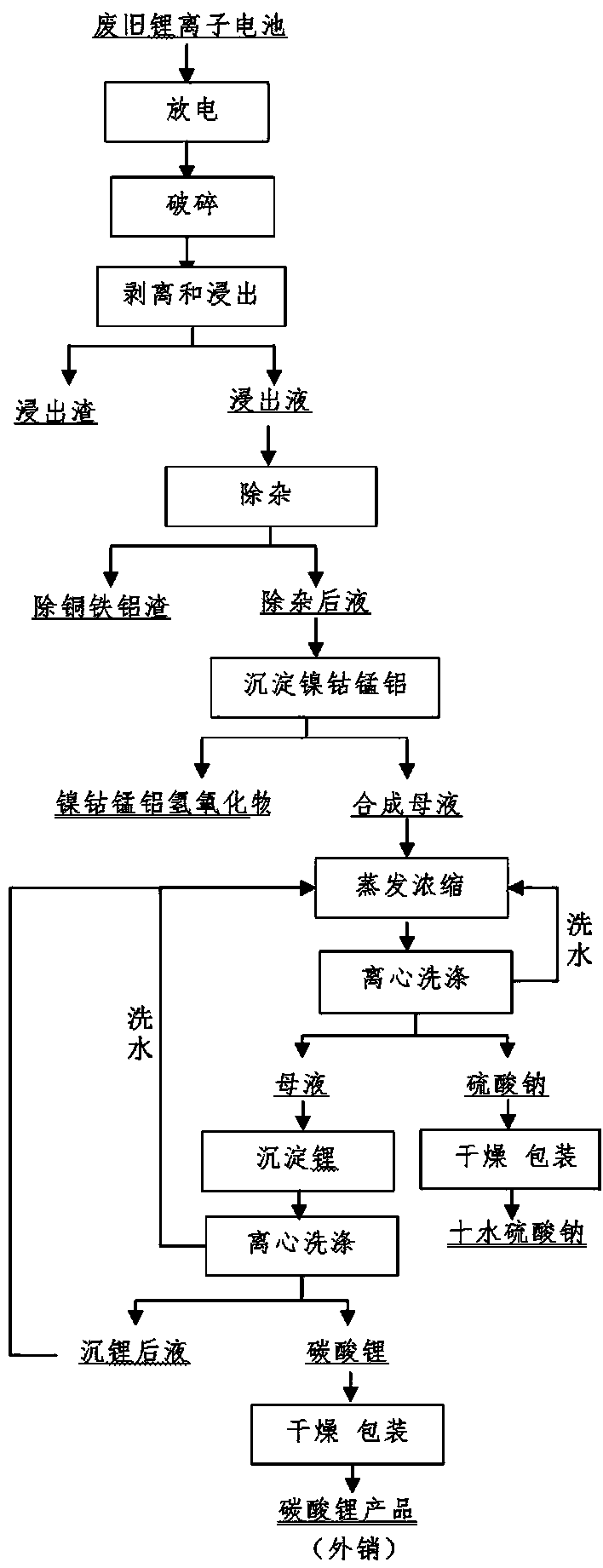
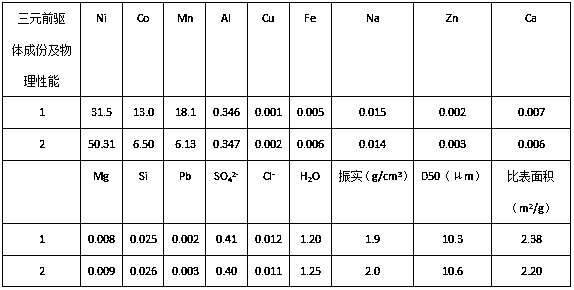
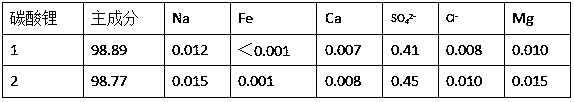
[0032] Collect waste lithium-ion batteries of lithium cobaltate, lithium manganate, and nickel-cobalt lithium manganate, disassemble them manually, discharge them in 5% sodium chloride solution, and crush them mechanically. Weigh 1500 g of anhydrous sodium sulfite and dissolve it for later use. Add 15L of 1.8mol / L sulfuric acid into the reactor and heat it to 50~65°C, weigh 5kg of the crushed waste lithium-ion battery and add it, stir at a low speed, measure a certain amount of 30~98% concentrated sulfuric acid to adjust the pH value of the solution 1.0 or so, add sodium sulfite solution dropwise after 10 minutes, react for 30~100 minutes after the dropwise addition, and filter. Heat the filtrate to 80~90°C, stop heating, add 250g / L sodium hydroxide solution to adjust the pH value of the solution to 2.0, add 10g of reduced iron powder, react for 15min, add 100mL of 30% hydrogen peroxide, react for about 20min, then add lye to adjust the solution The pH value is about 4.0, rea...
Embodiment 2
[0035] Collect waste lithium-ion batteries of lithium cobaltate, lithium manganate, and nickel-cobalt lithium manganate, disassemble them manually, discharge the batteries in 5% sodium chloride solution, and crush them mechanically. Add 15L of 2mol / L sulfuric acid to the reactor, raise the temperature to 50~65°C, weigh 5kg of the crushed waste lithium-ion battery and add it into it, soak for 10min, stir at a low speed, measure a certain amount of 98% concentrated sulfuric acid, and slowly add it to the reaction vessel , adjust the pH value of the solution to about 1.0, add 2.5L of 30% hydrogen peroxide, react for 30-100min after the dropwise addition, and filter. The filter residue was washed and dried, then ground, and passed through a 100-mesh sieve to obtain copper, aluminum foil and graphite powder. Heat the filtrate to 85°C, add 250g / L sodium hydroxide solution to adjust the pH value of the solution to 1.8, add 12g of reduced iron powder, react for 15min, add 30% hydrogen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com