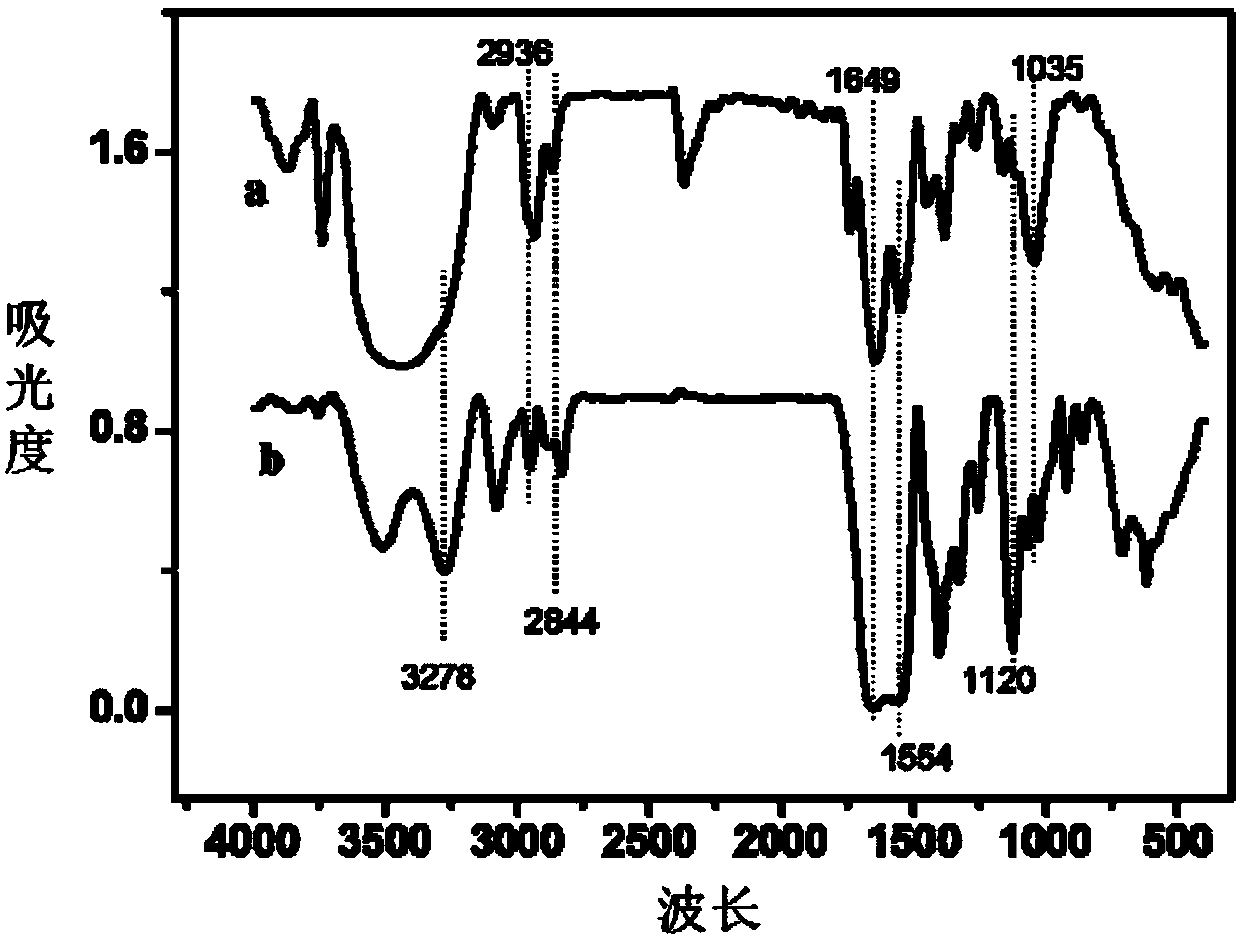
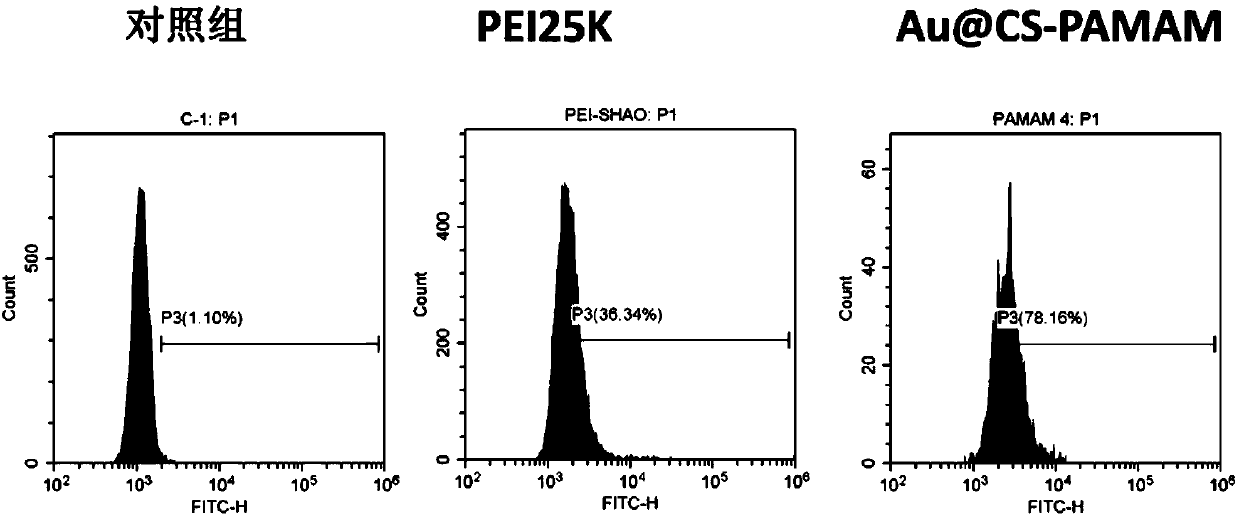
Dendritic macromolecule modified gold nano particles as well as preparation method and application thereof
A gold nanoparticle and dendritic technology, which is applied in the field of dendrimer-modified gold nanoparticle and its preparation, and achieves the effects of reducing biological toxicity, improving stability and easy structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] (1) Chitosan modified with azide group (CS-N 3 )Synthesis
[0063] Add azidoacetic acid (N 3 -CH2-COOH) was dissolved in NN-dimethylformamide (DMF), and then 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) and N-Hydroxysuccinimide (NHS) is activated for 30 minutes to obtain the azide acetic acid mixed solution; after the activation, chitosan (CS molecular weight is 2000, deacetylation degree 85%) is dissolved in pure water, and then slowly added to the above In the azide acetic acid mixed solution, the reaction was carried out at room temperature 35°C for 12 hours. The product was dialyzed in pure water for 2 days and freeze-dried to obtain the product azide chitosan; wherein, the chitosan, 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride The molar ratio of salt (EDC·HCl), N-hydroxysuccinimide (NHS) and azidoacetic acid is 1:1.5:1.5:2; the N,N-dimethylformamide is in 10 ml Add 5g hydrazoic acid; the pure water is calculated by adding 5g chi...
Embodiment 2
[0067] (1) Chitosan modified with azide group (CS-N 3 )Synthesis
[0068] Add azidoacetic acid (N 3 -CH2-COOH) was dissolved in NN-dimethylformamide (DMF), and then 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) and N-Hydroxysuccinimide (NHS) is activated for 4h to obtain the azide acetic acid mixed solution; after activation, chitosan (CS molecular weight is 20000, deacetylation degree 40%) is dissolved in pure water, and then slowly added to the above In the azide acetic acid mixed solution, react at room temperature for 24 hours. The product was dialyzed in pure water for 3 days and freeze-dried to obtain the product azide chitosan. Wherein, the chitosan, 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl), N-hydroxysuccinimide (NHS) and azidoacetic acid The molar ratio is 1:10:10:10; the N,N-dimethylformamide is calculated by adding 1g hydrazoic acid per 10ml; the pure water is calculated by adding 0.5g chitosan per 10ml Sugar meter. ...
Embodiment 3
[0072] (1) Chitosan modified with azide group (CS-N 3 )Synthesis
[0073] Add azidoacetic acid (N 3 -CH2-COOH) was dissolved in NN-dimethylformamide (DMF), and then 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) and N-Hydroxysuccinimide (NHS) is activated for 2h to obtain azide acetic acid mixed solution; after activation, chitosan (CS molecular weight is 10000, deacetylation degree 60%) is dissolved in pure water, and then slowly added to the above In the azide acetic acid mixed solution, react at room temperature for 16 hours. The product was dialyzed in pure water for 2 days and freeze-dried to obtain the product azide chitosan. Wherein, the chitosan, 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl), N-hydroxysuccinimide (NHS) and azidoacetic acid The molar ratio is 1:5:5:5; the N,N-dimethylformamide is calculated by adding 3g hydrazoic acid per 10ml; the pure water is calculated by adding 3g chitosan per 10ml meter.
[0074] (2) Syn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com