Double photo-responsive aluminum oxide nano channel based on N3 and spiropyrane molecular modification and preparation method thereof
A technology of alumina nano and spiropyran molecules, applied in nanostructure manufacturing, nanotechnology, nano optics, etc., can solve the problems of low mechanical properties, poor repeatability of channel materials, poor stability, etc., and achieve the effect of current amplification characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] This embodiment is an hourglass-type alumina nanochannel system. The hourglass-type alumina nanochannel film is prepared by an oxalic acid solution anodizing method, the oxidation voltage is 50V, and the hourglass-type structure is obtained by in-situ expansion using current monitoring; The thickness of the hourglass-type alumina nanochannel film is about 98um; the pore diameter at both ends of the hourglass-type alumina nanochannel film is about 35nm, and the middle pore diameter is about 10nm.
[0037] The preparation of the hourglass alumina nanochannel adopts a combination of double-sided anodization and in-situ hole expansion. The specific operation steps are as follows:
[0038] (1) A flat aluminum sheet with a purity of 99.999% and a thickness of about 100 μm is ultrasonically cleaned with acetone, absolute ethanol and high-purity water for 5 minutes, and then electrochemically polished in a mixture of perchloric acid and ethanol (1:4). The polishing time was 5 minute...
Embodiment 2
[0045] A solution method is used to modify the APTES molecules on the hourglass-type alumina nanochannel.
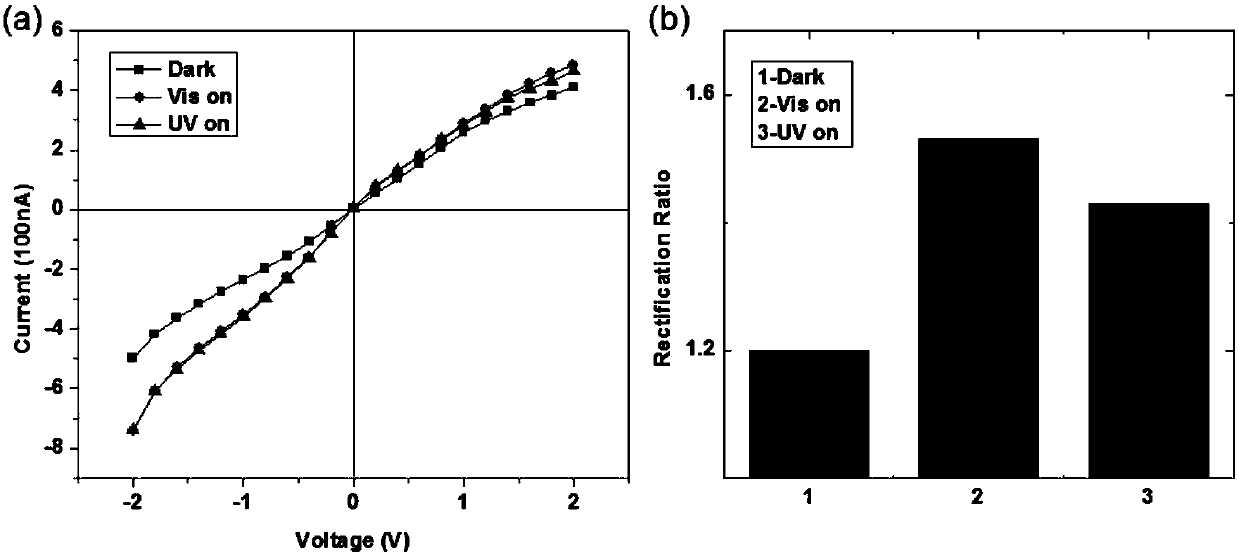
[0046] The picoammeter was used as the control circuit to perform IV performance test on the hourglass alumina nanochannel modified by the APTES molecule; wherein the electrolyte solution was a KCl solution with a concentration of 1 mM / L and the pH of the solution was 3; the APTES molecule was modified The voltage scanning range of the hourglass alumina nanochannel is from -2V to +2V, and it is concluded that the ion current fluctuates with the fluctuation of the applied voltage, such as figure 1 As shown, the tested I-V curve is linear, so the alumina nanochannel modified by the APTES molecule has no ion rectification characteristics.
[0047] The contact angle measurement of the alumina nanochannel and the alumina nanochannel modified with APTES shows that the bare-hole alumina nanochannel is more hydrophilic ( figure 1 In the middle b and c are the contact angles of the upp...
Embodiment 3
[0049] A solution method is used to perform single-sided modification of N3 molecules on the alumina nanochannel modified by APTES.
[0050] Using the picoammeter as the control circuit, the IV performance test was performed on the hourglass-type alumina nanometer modified on one side of the N3 molecule; wherein the electrolyte solution was a KCl solution with a concentration of 1 mM / L and a pH of 3; the N3 The voltage sweep range of the hourglass alumina nanochannel modified on one side of the molecule is from -2V to +2V, and the results are as follows figure 2 , It is concluded that the ion current fluctuates with the fluctuation of the applied voltage, and the tested IV curve is non-linear. Therefore, the hourglass-type alumina nanochannel modified by the single side of the N3 molecule exhibits ion rectification characteristics, that is, the voltage is at -2V In the negative voltage region to +0V, a larger current is exhibited, and the hourglass alumina nanochannel exhibits an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com