Lithium Complex Oxide For Lithium Secondary Battery Positive Active Material And Method Of Preparing The Same
A composite oxide, lithium secondary battery technology, used in secondary batteries, lithium storage batteries, chemical instruments and methods, etc., can solve the problems of high LiOH viscosity, decreased capacity and rate characteristics, and surface damage of positive active materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Preparation of positive electrode active material
[0067] First, NiCo(OH) was prepared by co-precipitation reaction 2 Precursor. Add Li to the prepared precursor 2 CO 3 LiOH is used as a lithium compound, and Al and Mg are added as M1, and then the first heat treatment is performed to prepare a positive electrode active material for a lithium secondary battery.
[0068] Prepare distilled water, put the prepared positive electrode active material for lithium secondary battery into the distilled water, and wash the prepared positive electrode active material for lithium secondary battery while maintaining the temperature.
[0069] Then, 0.03 mole of cobalt sulfate aqueous solution is dropped into the positive electrode active material washing solution in a certain proportion, and the positive electrode active material is stirred at the same time, and the input time is 1 hour, thereby using Co as M2 The surface of the positive electrode active material is coated and...
Embodiment 2
[0078] Preparation of positive electrode active material
[0079] The positive electrode active material of Example 2 was prepared by performing the same washing and coating procedures as in Example 1 above, except that a 4 mol% cobalt aqueous solution was added to the washing solution of the positive electrode active material.
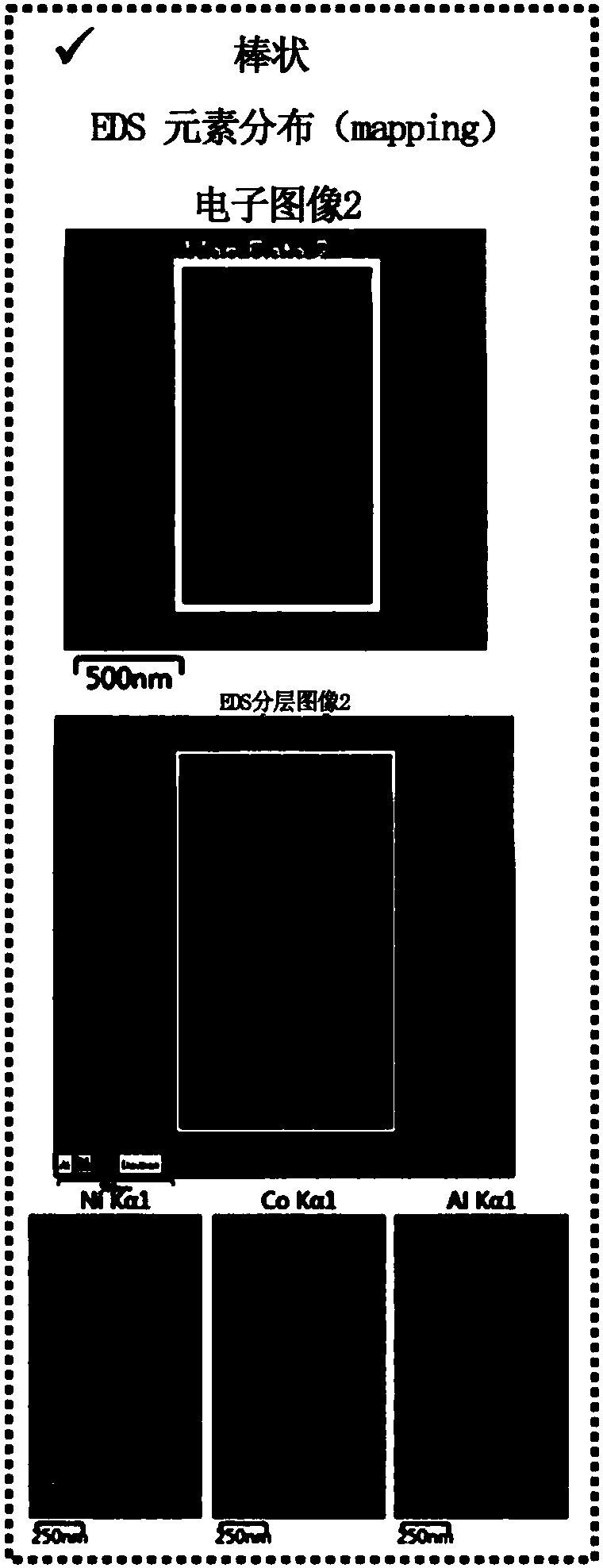
[0080] TEM and EDX measurement
[0081] Measure the TEM and EDX photographs of the positive electrode active material prepared in described embodiment 2, and the measurement results are shown in Figure 4 .
[0082] Such as Figure 4 It can be seen that, for the positive electrode active material prepared in Example 2 of the present invention, the Co concentration on the surface of the secondary particle is relatively high, and the Co concentration gradually decreases toward the inside of the secondary particle in a gradient distribution, rather than constant.
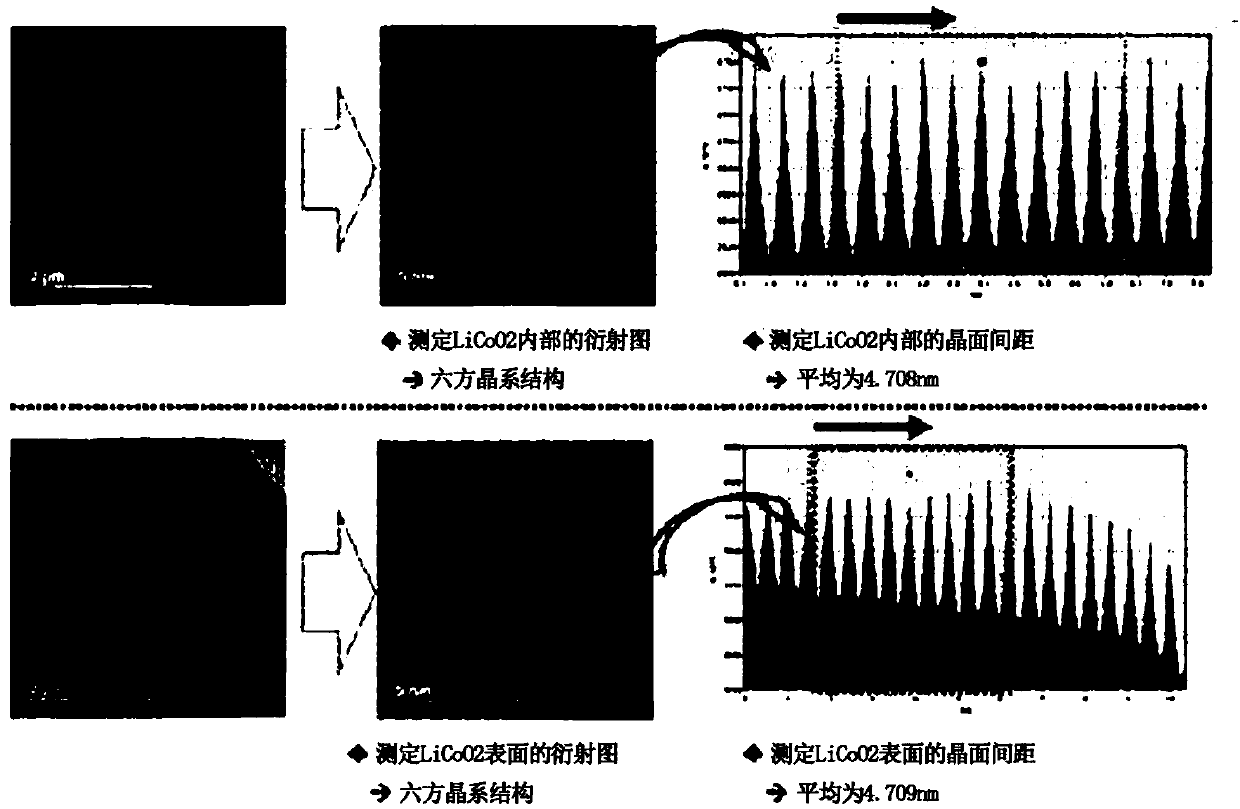
[0083] Measurement of interplanar spacing of crystal structure
[0084] For the...
Embodiment 3
[0087] Preparation of NCM-based positive electrode active material
[0088] The positive electrode active material of Example 3 was prepared in the same manner as in Example 1 above, except that a 5 mol% cobalt aqueous solution was added to the washing solution of the positive electrode active material for coating.
[0089] Scanning density
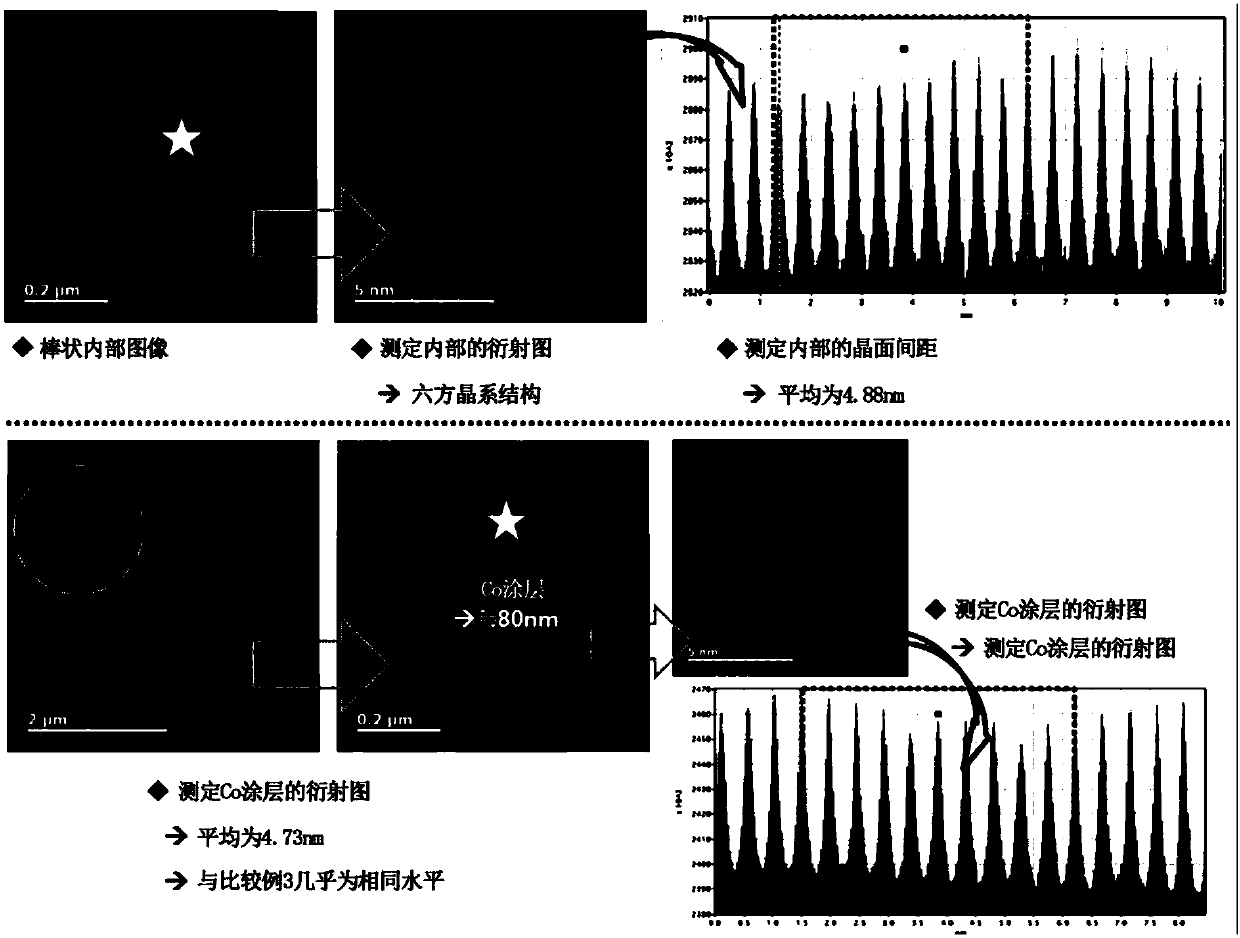
[0090] Measure the change of each concentration of Ni, Co and Al in the positive electrode active material prepared in said embodiment 3 from the secondary particle surface to the direction of the particle center, and the measurement results are shown in Figure 6 .
[0091] From Figure 6 It can be seen that for the positive electrode active material prepared in Example 3 of the present invention, the Co concentration on the Co coating gradually increases from the surface to the center, but then the Co concentration gradually decreases to the center, and the thickness of the Co coating is 0.1 μm.
[0092] TEM and EDX measureme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com