Moroxydine hydrochloride purity standard substance as well as preparation method and application thereof
A kind of technology of morpholine guanidine hydrochloride and standard substance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
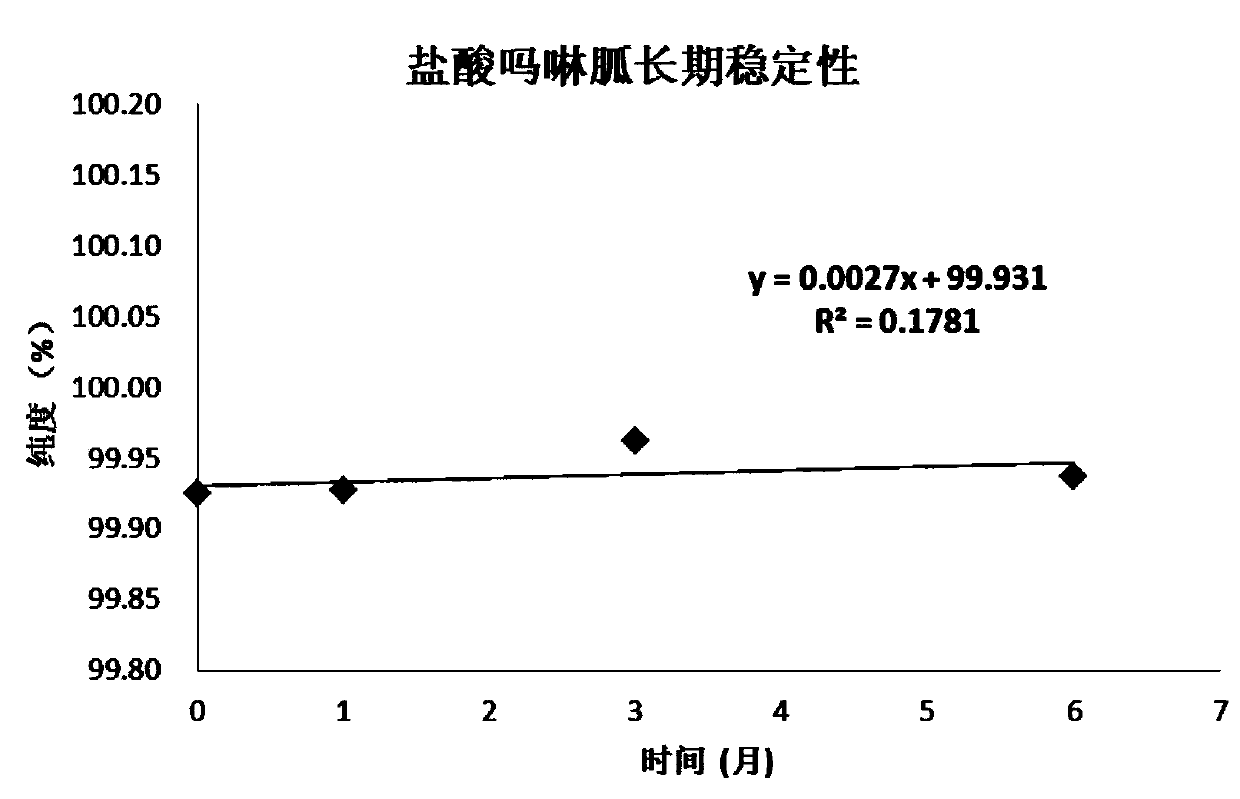
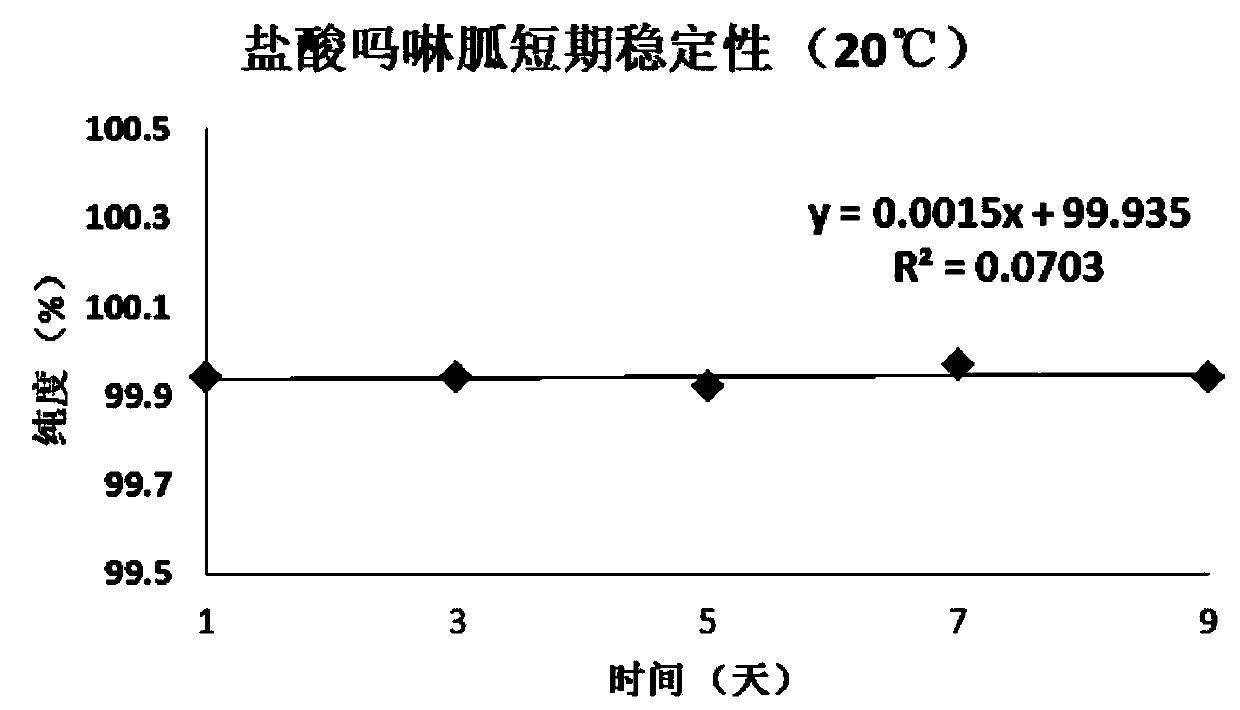
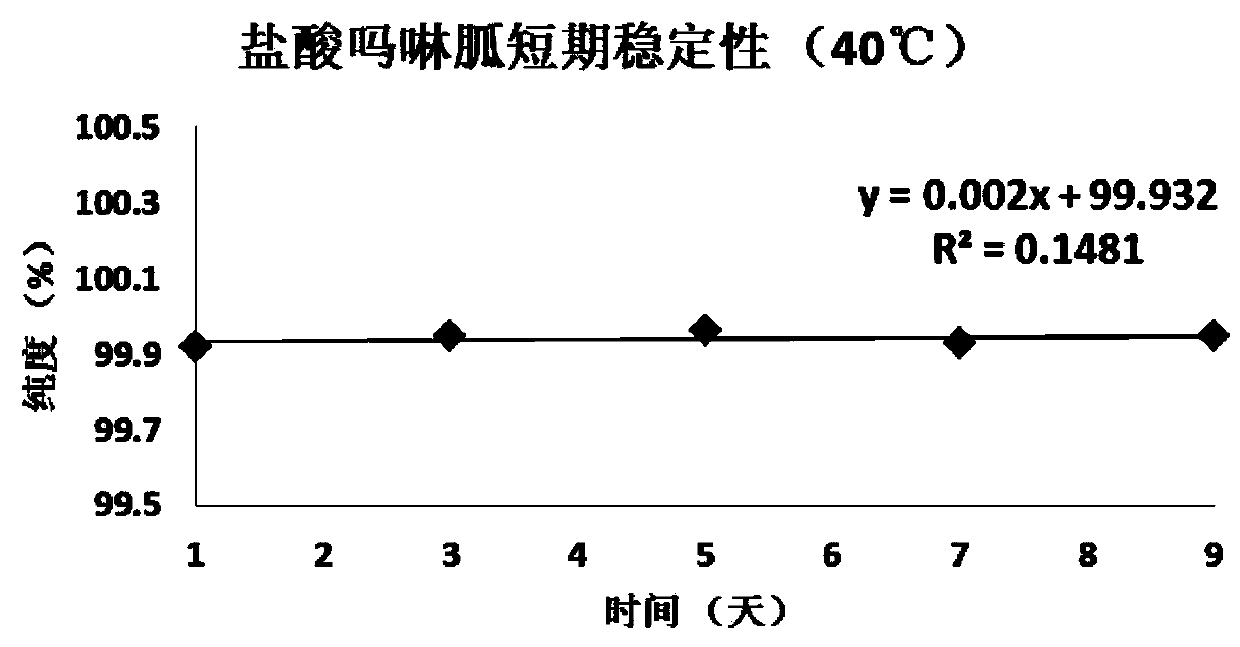
[0058] Embodiment 1, the definite value of morpholinidine hydrochloride purity standard substance
[0059] Preparation of morpholine guanidine hydrochloride raw material powder: After the pure morpholine guanidine hydrochloride raw material is through infrared and NMR after carrying out qualitative and purity initial confirmation, be placed in the grinder and fully grind, the raw material after grinding is put into vacuum oven, in 45 Under the condition of ℃, dry at low pressure (below -0.1Mpa) for 24 hours, place the raw material of pure morpholine guanidine hydrochloride dried under reduced pressure in a clean glass vessel, stir and mix well. Adopt liquid chromatography (ultraviolet detector, UV) to carry out the preliminary inspection of homogeneity to this raw material, after the inspection is qualified, the morpholine guanidine hydrochloride candidate is sub-packed in clean vials, and the vial specification is 5.0mL, in Strict washing measures are used to clean before sub...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com