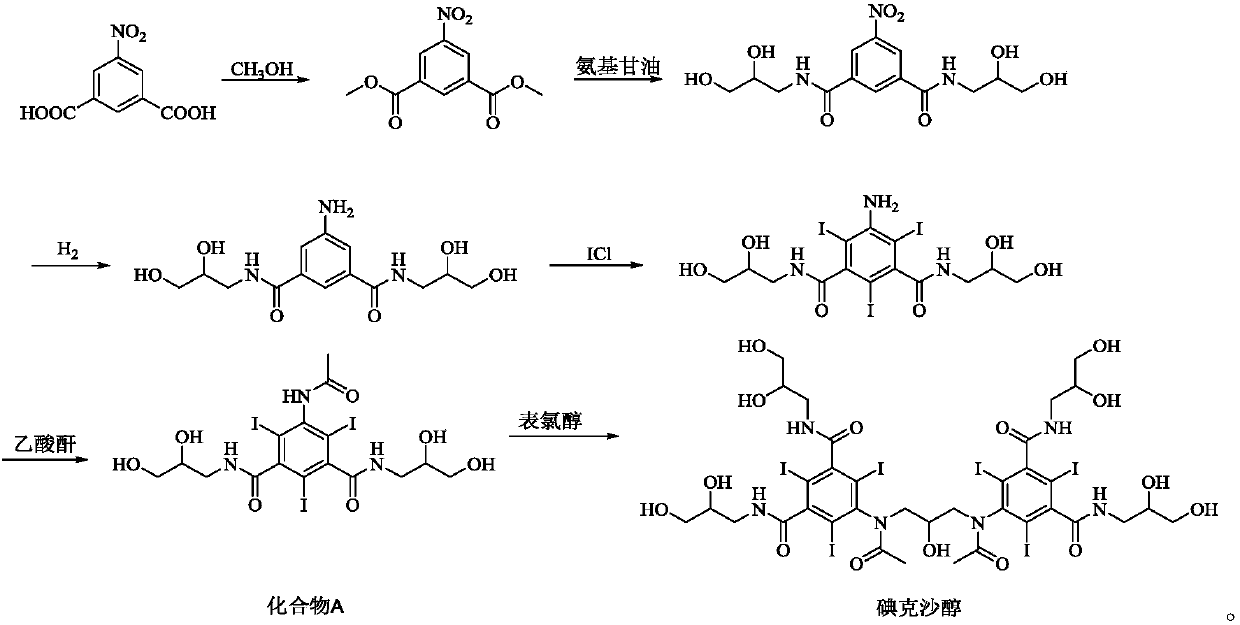
Crystallization purification method for iodixanol
A purification method, iodixanol technology, applied in the separation/purification of carboxylic acid amide, organic chemistry, etc., can solve the problems of long recrystallization time and difficult removal of crystallization solvent, and achieve short time consumption, less solvent residue, and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 50g of crude iodixanol (moisture: 3.0%, HPLC purity: 97.8%) was dissolved in 200mL of methanol, refluxed and stirred to dissolve, 1.5g of activated carbon was added and refluxed for 0.5h, filtered, the filtrate continued to refluxed for 15min, and slowly added 20mL of Water and ethanol, continue to reflux for 15 hours, cool to 35°C, filter, wash the filter cake with a small amount of methanol, blow dry at 80°C for 5-10 hours, then weigh 41g, the yield is 82%, and the HPLC purity is 99.7%. Solvent residue: Methanol was not detected, ethanol 8ppm.
Embodiment 2
[0037] 500g of crude iodixanol (moisture: 2.8%, HPLC purity: 96.8%) was dissolved in 2.5L of methanol, refluxed and stirred to dissolve, added 15g of activated carbon and continued to reflux for 0.5h, filtered, the filtrate continued to reflux for 0.5h, and slowly added 400mL After the addition of absolute ethanol, continue to reflux for 20 hours, cool down to 35°C, filter, wash the filter cake with a small amount of methanol, blow dry at 80°C for 5-10 hours, and then weigh 400g. The yield is 80%, and the HPLC purity is 99.5%. Solvent residue: methanol 2ppm, ethanol 10ppm.
Embodiment 3
[0039] 5Kg of crude iodixanol (moisture: 2.5%, HPLC purity: 97.3%) was dissolved in 25L of methanol, refluxed and stirred to dissolve, added 150g of activated carbon and continued to reflux for 0.5h, filtered, and the filtrate continued to reflux for 0.5h, and slowly added 5L of After adding water and ethanol, add 2g of seed crystals (seed crystals from Example 2), continue to reflux for 20h, cool to 35°C, filter, wash the filter cake with a small amount of methanol, weigh 4.1Kg after drying, and the yield is 82%, HPLC The purity is 99.5%. Solvent residue: methanol 3ppm, ethanol 10ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com