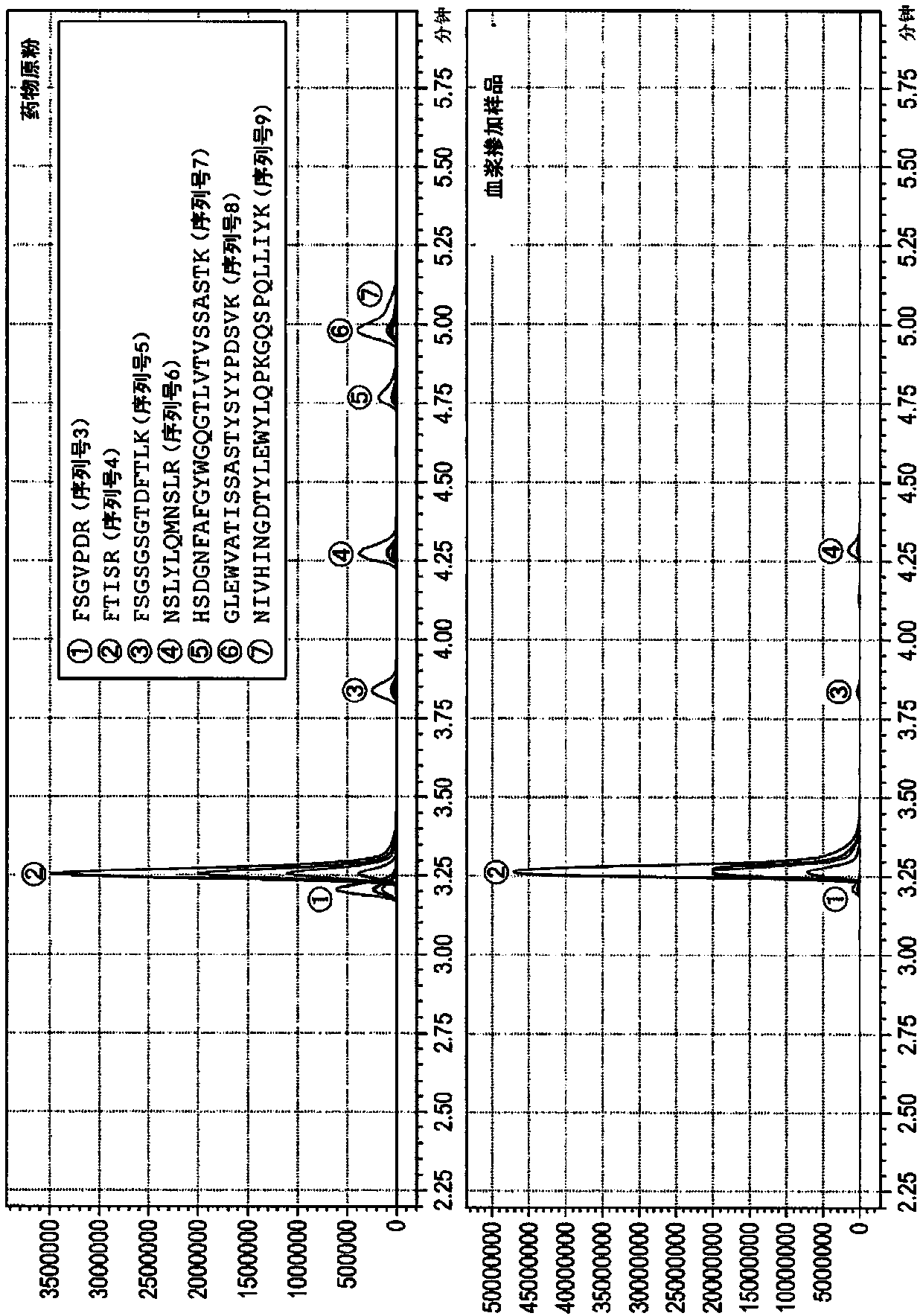
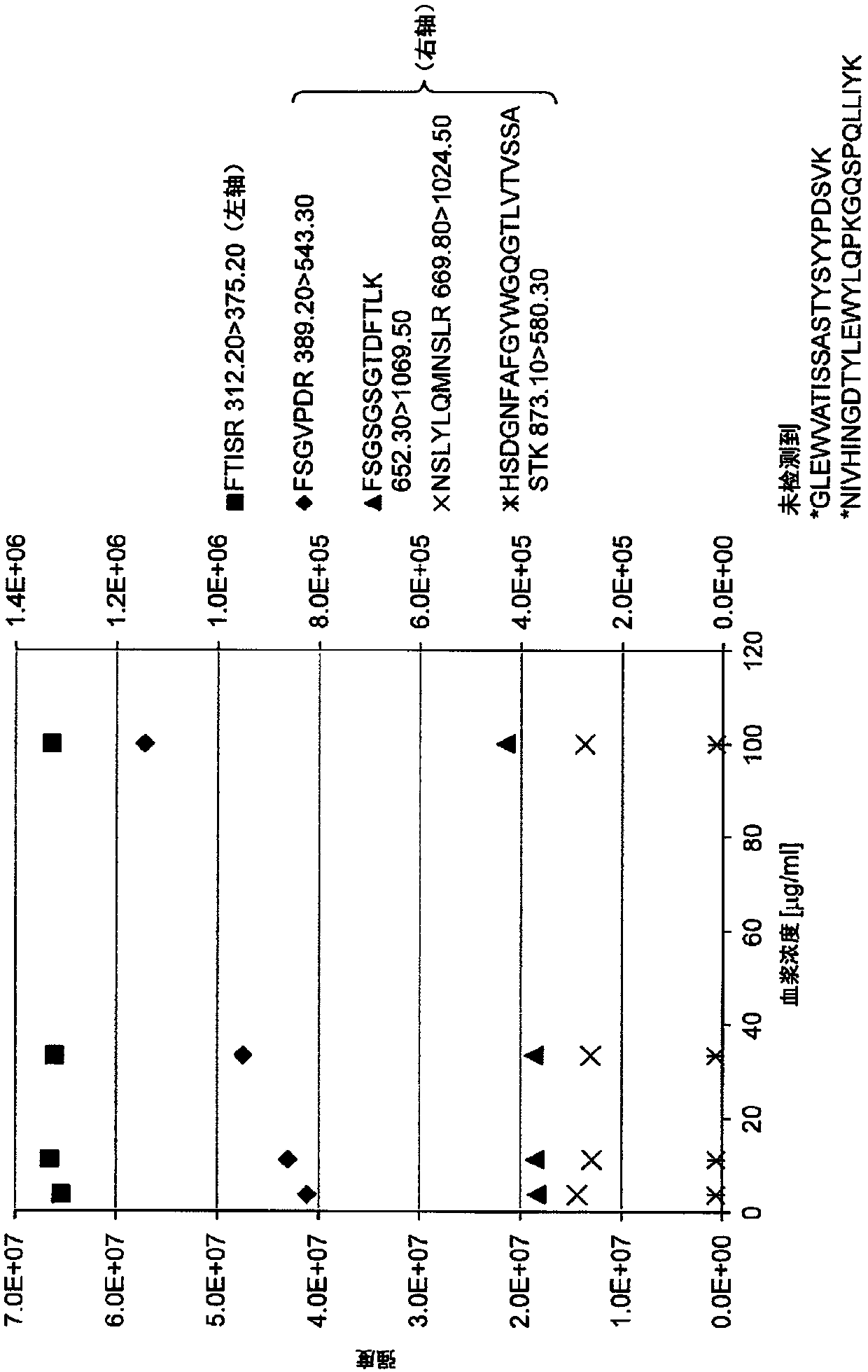
Method for quantifying monoclonal antibody
A monoclonal antibody and porous body technology, applied in biochemical equipment and methods, microbe determination/inspection, measuring devices, etc., can solve time-consuming and cost-intensive problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0172] The present invention will be further specifically described based on the following examples, but the present invention is not limited by the examples.
[0173] [Immobilization of protease]
[0174] Protease was immobilized on microparticles by the following steps.
[0175] 1. FG bead washing
[0176] 200 mg of FG beads NHS (manufactured by Tamagawa Seiki Co., Ltd.) was centrifuged (15000 g x 5 minutes, 4° C.), and the supernatant for storage was removed with isopropanol. The supernatant also contains sediment and even floating matter, which should be carefully removed.
[0177] 2. Enzyme Preparation
[0178] 1 mg of Trypsin Gold (manufactured by Promega Corporation) or 5 mg of Trypsin TPCK (manufactured by Sigma-Aldrich Co. LLC.) was opened and dissolved in 25 mM HEPES-NaOH, pH 7.0, cooled to 4°C. After dissolving, transfer to a 50ml centrifuge tube and place on ice. The enzyme was co-washed once with 25mM HEPES-NaOH, pH 7.0, and recovered as much as possible. Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com