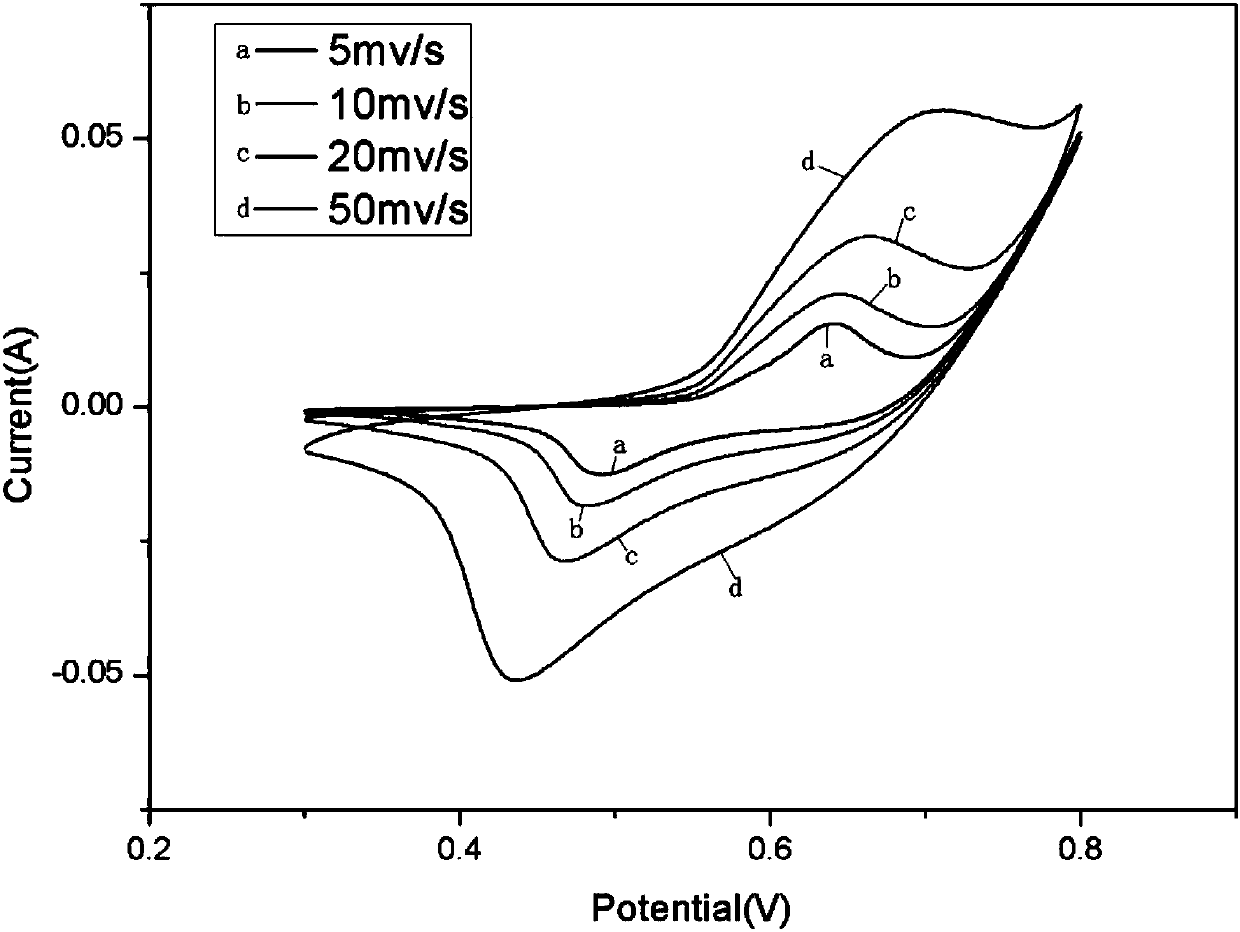
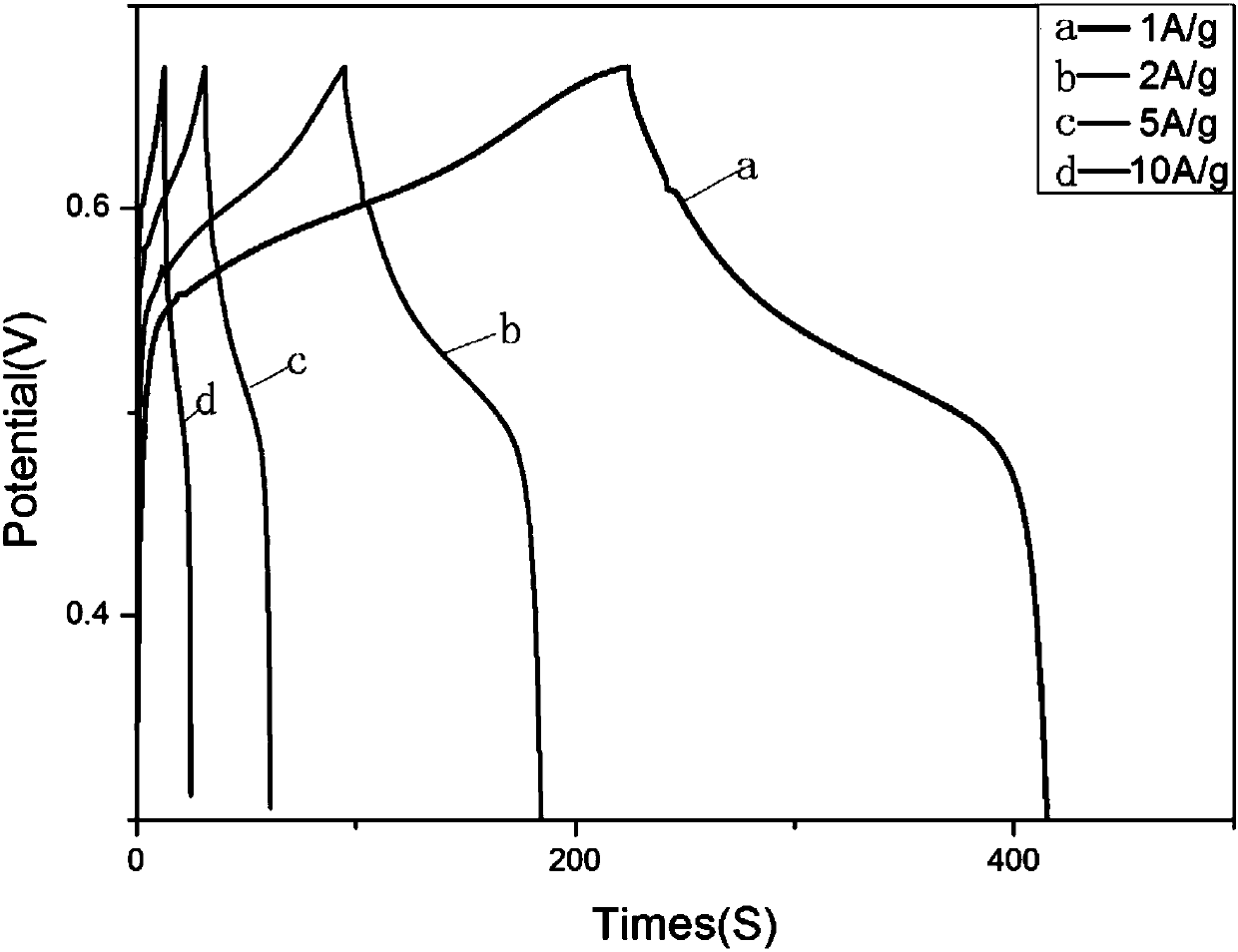
Polyhedron bimetallic oxide as well as preparation method and application thereof
A bimetallic oxide, polyhedron technology, applied in chemical instruments and methods, oxygen/ozone/oxide/hydroxide, non-metallic elements, etc., can solve the problems of excessive crystallinity, high price, small specific surface area, etc. , to achieve the effect of mild conditions, controllable operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Synthesis of Fe-containing zif-67
[0027] Weigh 0.4774g Co(NO 3 ) 2 ·6H 2 O and 0.331g Fe(NO 3 ) 2 ·6H 2 O was dissolved in 50ml deionized water to form solution A; 1.622g 2-methylimidazole and 3ml triethylamine were dissolved in 50ml deionized water to form solution B; solution A and solution B were stirred evenly and mixed respectively; wherein, Co (NO 3 ) 2 ·6H 2 O+Fe(NO 3 ) 2 ·6H 2 The molar ratio of O, 2-methylimidazole and triethylamine was 1:8:8; the mixed solution was stirred evenly at room temperature and allowed to stand for 24 hours, and then centrifuged. Centrifuge and wash with deionized water for 2-3 times, and dry in an oven at a temperature of 70-100°C for 12-24 hours to obtain iron-containing zif-67 powder;
[0028] 2. Preparation of polyhedral double metal oxide materials
[0029] The obtained iron-containing zif-67 powder was oxidized in a tube furnace at 350° C. for 3 hours, and oxidized in air to obtain a polyhedral double metal oxi...
Embodiment 2
[0034] 1. Synthesis of nickel-containing zif-67
[0035] Weigh 0.4774g Co(NO 3 ) 2 ·6H 2 O and 0.238g Ni(NO 3 ) 2 ·6H 2O was dissolved in 50ml deionized water to form solution A; 1.622g 2-methylimidazole and 3ml triethylamine were dissolved in 50ml deionized water to form solution B; solution A and solution B were stirred evenly and mixed respectively; wherein, Co (NO 3 ) 2 ·6H 2 O+Ni(NO 3 ) 2 ·6H 2 The molar ratio of O, 2-methylimidazole and triethylamine was 1:8:8; the mixed solution was stirred evenly at room temperature and allowed to stand for 24 hours, and then centrifuged. Centrifuge and wash with deionized water for 2-3 times, and dry in an oven at a temperature of 70-100°C for 12-24 hours to obtain iron-containing zif-67 powder;
[0036] 2. Preparation of polyhedral double metal oxide materials
[0037] The obtained Ni-containing zif-67 powder was oxidized in a tube furnace at 350° C. for 3 hours, and then oxidized in air to obtain a polyhedral double met...
Embodiment 3
[0041] 1. Synthesis of iron-containing zif-8
[0042] Weigh 0.488g Zn(NO 3 ) 2 ·6H 2 O and 0.331g Fe(NO 3 ) 2 ·6H 2 O was dissolved in 50ml of deionized water to form solution A; 1.622g of 2-methylimidazole and 3ml of triethylamine were dissolved in 50ml of deionized water to form solution B; solution A and solution B were stirred evenly and then mixed; wherein, Zn (NO 3 ) 2 ·6H 2 O+Fe(NO 3 ) 2 ·6H 2 The molar ratio of O, 2-methylimidazole and triethylamine was 1:8:8; the mixed solution was stirred evenly at room temperature and allowed to stand for 24 hours, and then centrifuged. Centrifugal washing with deionized water for 2-3 times, drying in an oven at a temperature of 70-100° C. for 12-24 hours to obtain iron-containing zif-8 powder.
[0043] 2. Preparation of polyhedral double metal oxide materials
[0044] The obtained Fe-containing zif-8 powder was oxidized in a tube furnace at 350° C. for 3 hours, and then oxidized in air to obtain a polyhedral double met...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Charge and discharge current density | aaaaa | aaaaa |
| Specific capacitance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com