Enzalutamide pharmaceutical composition and preparation method thereof
A technology of enzalutamide and composition, applied in the field of enzalutamide pharmaceutical composition and preparation thereof, can solve the problems of incompleteness, low bioavailability, low solubility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
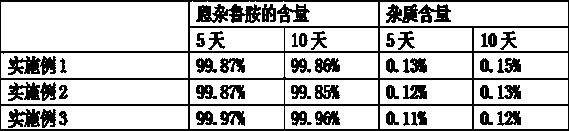
Embodiment 1
[0033] Enzalutamide 20g
[0034] Hydroxypropyl-β-cyclodextrin 60g
[0035] Mannitol 20g
[0036] Microcrystalline Cellulose 20g
[0037] Croscarmellose Sodium 5g
[0038] 2% polyvinylpyrrolidone aqueous solution 100g
[0040] Preparation Process:
[0041] Take the prescribed amount of hydroxypropyl-β-cyclodextrin and dissolve it in an appropriate amount of water to make a saturated aqueous solution. Pass enzalutamide through a 120 mesh sieve and dissolve it in an appropriate amount of ethanol. Under magnetic stirring, the ethanol solution of enzalutamide Slowly add the saturated aqueous solution of hydroxypropyl-β-cyclodextrin, continue stirring at room temperature for 3 hours after all the addition is complete, and remove most of the ethanol by rotary evaporation at 45°C. Put it into a shallow tray and put it in the freezer of the refrigerator. The pre-freezing time is 12 hours and the temperature is -50°C. When the temperature of the cold...
Embodiment 2
[0045] Enzalutamide 20g
[0046] Hydroxypropyl-β-cyclodextrin 20g
[0047] Mannitol 15g
[0048] Microcrystalline Cellulose 15g
[0049] Croscarmellose Sodium 2g
[0050] 2% polyvinylpyrrolidone aqueous solution 100g
[0052] Its preparation process is with embodiment 1.
Embodiment 3
[0054] Enzalutamide 20g
[0055] Hydroxypropyl-β-cyclodextrin 40g
[0056] Mannitol 25g
[0057] Microcrystalline Cellulose 25g
[0058] Croscarmellose Sodium 5g
[0059] 2% polyvinylpyrrolidone aqueous solution 100g
[0061]Its preparation process is with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com