Memantine hydrochloride sustained-release pellet and preparation method thereof
A technology of memantine hydrochloride and sustained-release pellets, applied in the direction of pharmaceutical formulations, microcapsules, medical preparations of non-active ingredients, etc., to achieve the effect of ensuring stability and complete release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
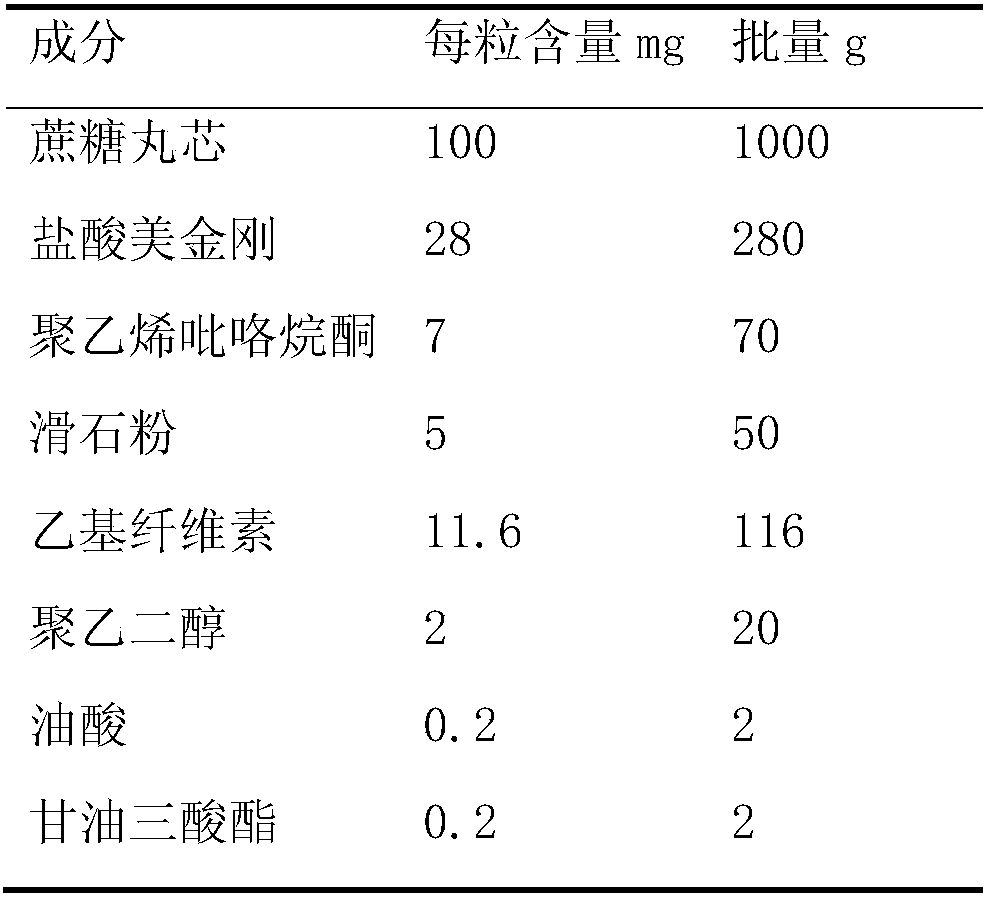
[0026] Embodiment 1: Preparation of memantine sustained-release pellets
[0027]
[0028] Preparation of drug-containing pellets: use 1600g of 50% ethanol aqueous solution as the drug dispersion medium, add 70g of polyvinylpyrrolidone, then disperse 280g of memantine hydrochloride and 50g of talc in the polyvinylpyrrolidone solution to obtain a suspension; adopt a fluidized bed Bottom spraying method, at a temperature of 30-40°C, spray the drug-on-drug suspension onto the blank pellet core to obtain drug-containing pellets.
[0029] Sustained-release pellet preparation: take 1260g 95% ethanol aqueous solution as the dispersion medium of the sustained-release layer, add 2g oleic acid, 2g triglyceride, then add 20g polyethylene glycol, stir until dissolved and then add 116g ethyl cellulose to obtain the sustained-release pellet. release layer suspension; using the fluidized bed bottom spray method, spray the sustained release layer suspension onto the drug-containing pellets ...
Embodiment 2
[0030] Embodiment 2: Preparation of memantine sustained-release layer pellets
[0031]
[0032]
[0033] Preparation of drug-containing pellets: preparation of drug-containing pellets in the same manner as in Example 1.
[0034] Preparation of sustained-release pellets: Add 373g of water to 560g of Surelease aqueous dispersion, and stir to obtain a sustained-release layer suspension; use the fluidized bed bottom spray method, mix the prepared sustained-release layer at a temperature of 40-50°C The suspension is sprayed onto the drug-containing pellets to obtain sustained-release layer pellets; the pellets are dried on-line in a fluidized bed at 40-60°C for 0.5-2 hours; the content is measured, and the pellets are filled into corresponding capsules to obtain the product.
Embodiment 3
[0035] Embodiment 3: Preparation of memantine sustained-release pellets
[0036]
[0037] Preparation of drug-containing pellets: preparation of drug-containing pellets in the same manner as in Example 1.
[0038] Preparation of extended-release pellets:
[0039] Sustained-release layer pellet 1: take 630g 95% ethanol aqueous solution as the dispersion medium of the sustained-release layer, add 1g oleic acid, 1g triglyceride, then add 10g polyethylene glycol, stir until dissolved, then add 58g ethyl cellulose to obtain Slow release layer suspension. Using a fluidized bed bottom spraying method, spray the prepared sustained-release layer suspension onto drug-containing pellets at a temperature of 30-40°C to obtain sustained-release layer pellets 1;
[0040] Sustained-release layer pellets 2: Add 187g of water to 280g of Surelease aqueous dispersion, and stir to obtain a sustained-release layer suspension; the prepared sustained-release layer is sprayed at the temperature o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com