Technology for extracting lithium from alkaline bittern
A technology for extracting lithium and brine, which is applied in the field of lithium extraction, can solve the problems of emulsification failure of extractant, complex process flow, poor extraction effect, etc., and achieve the effects of avoiding the small difference in density between the two phases, high operational reliability, and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] to Li + The concentration is 2g / L, Na + Add 2 mol / L sodium hydroxide solution to the chloride-type brine with a concentration of 20 g / L, adjust the pH value to 8.5-11.0, and obtain the extracted aqueous phase.
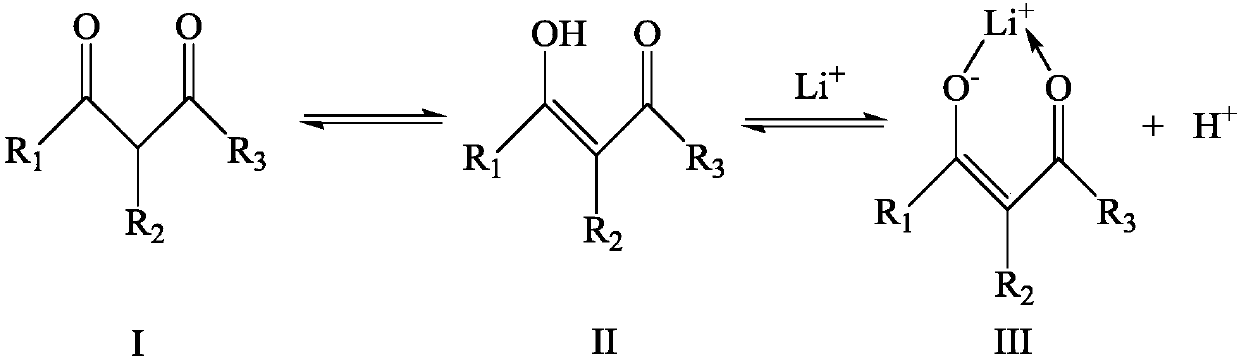
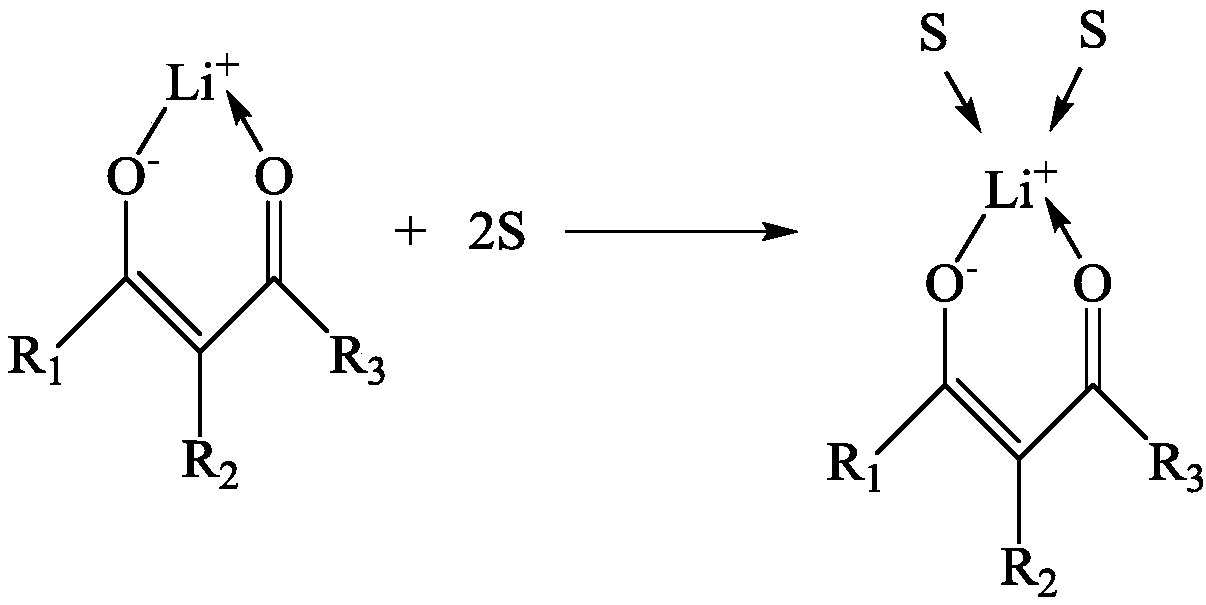
[0066] Benzoyltrifluoroacetone (hereinafter referred to as HBTA), trioctylphosphine oxide (hereinafter referred to as TOPO) and kerosene are mixed to obtain an extracted organic phase; wherein, in the extracted organic phase, the concentration of HBTA and TOPO is 0.4mol / L.
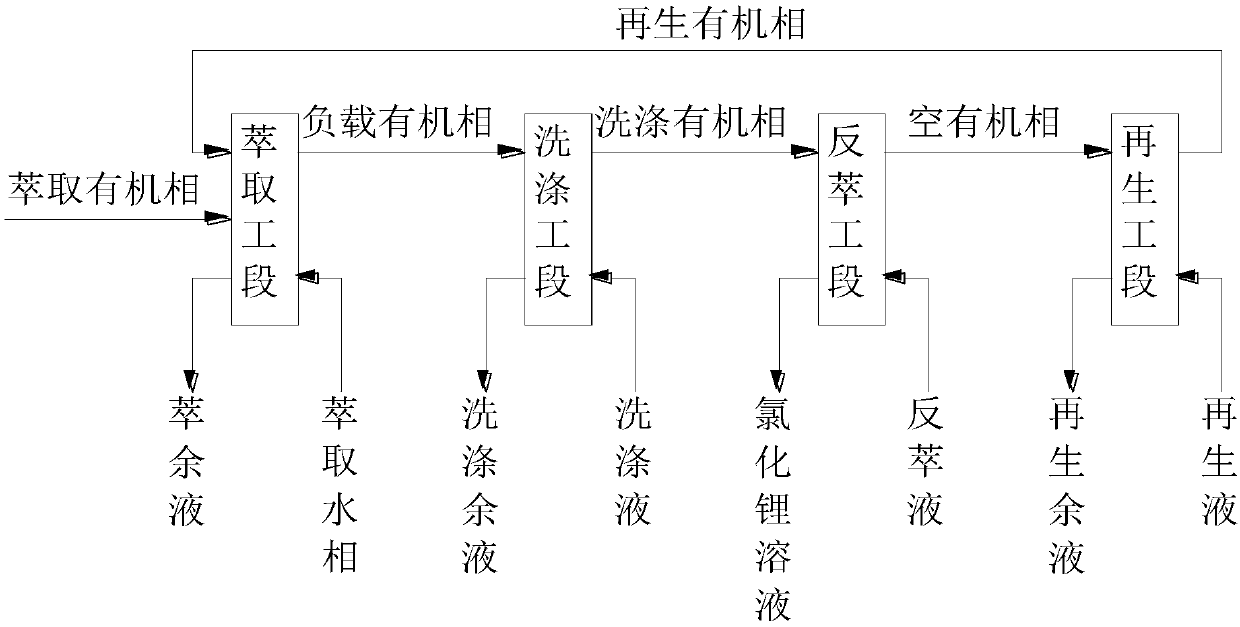
[0067] The extracted organic phase and the extracted aqueous phase prepared above were mixed according to the extraction ratio of 2:3, and three-stage countercurrent extraction was performed, and the single-stage extraction time was controlled to be 6 minutes. After the extraction equilibrium was reached, the raffinate was obtained by static phase separation. liquid and loaded organic phase.
[0068] Using 1mol / L-4mol / L hydrochloric acid solution as the washing solution, carry out three-s...
Embodiment 2
[0074] to Li + The concentration is 2g / L, Na + Add 2 mol / L sodium hydroxide solution to the carbonate brine with a concentration of 40 g / L to adjust its pH value to 10.0 to obtain the extracted aqueous phase.
[0075] Mix HBTA, TOPO and kerosene to obtain an extracted organic phase; wherein, in the extracted organic phase, the concentration of HBTA and TOPO are both 0.3 mol / L.
[0076] The extracted organic phase and the extracted aqueous phase prepared above were mixed according to the extraction ratio of 1:1, and three-stage countercurrent extraction was performed, and the single-stage extraction time was controlled to be 6 minutes. After the extraction equilibrium was reached, the raffinate was obtained by static phase separation. liquid and loaded organic phase.
[0077] Using 2mol / L hydrochloric acid solution as the washing solution, carry out three-stage countercurrent washing on the above-mentioned loaded organic phase, control the washing ratio to 15:1, and the singl...
Embodiment 3
[0083] to Li + The concentration is 2g / L, Na + Add 2 mol / L sodium carbonate solution to the sulfate brine with a concentration of 60 g / L to adjust the pH value to 11.0 to obtain the extracted aqueous phase.
[0084] Mix HBTA, TOPO and kerosene to obtain an extracted organic phase; wherein, in the extracted organic phase, the concentration of HBTA and TOPO are both 0.3 mol / L.
[0085] The extracted organic phase and the extracted aqueous phase prepared above were mixed according to the extraction ratio of 1:1, and three-stage countercurrent extraction was performed, and the single-stage extraction time was controlled to be 6 minutes. After the extraction equilibrium was reached, the raffinate was obtained by static phase separation. liquid and loaded organic phase.
[0086] Using 1mol / L hydrochloric acid solution as the washing solution, carry out three-stage countercurrent washing on the above-mentioned loaded organic phase, control the washing ratio to 20:1, and the single-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com