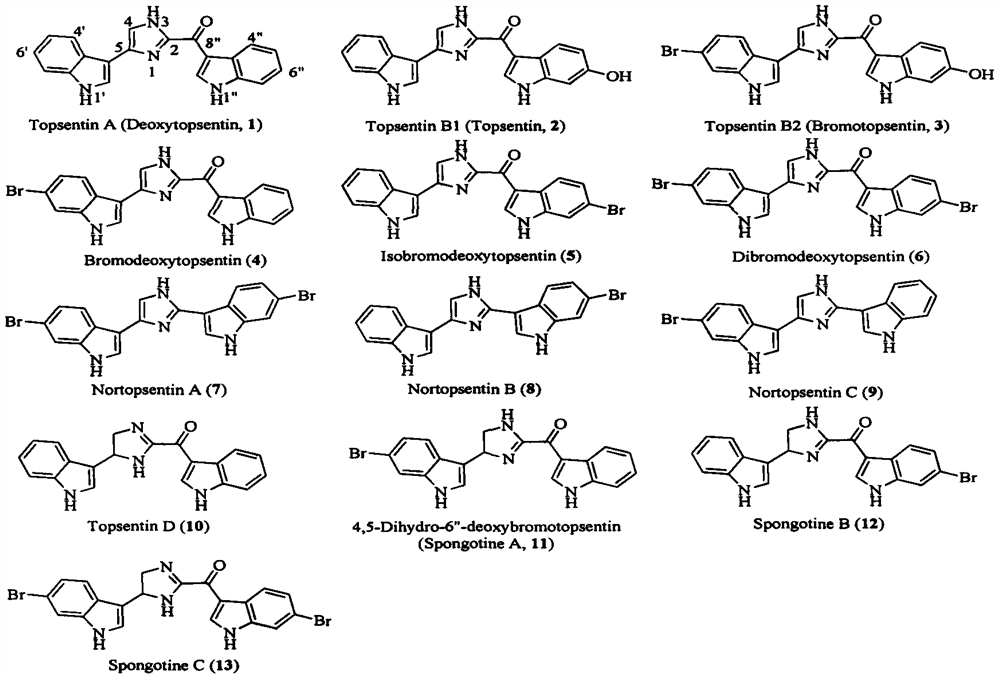
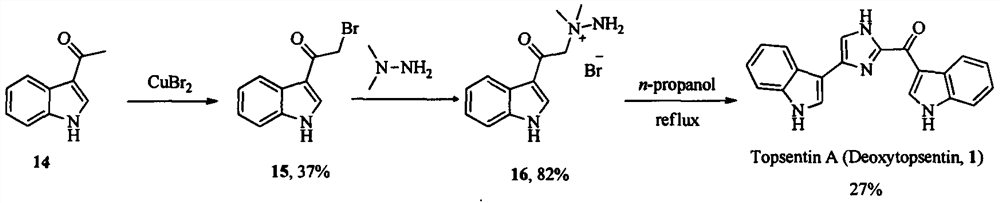
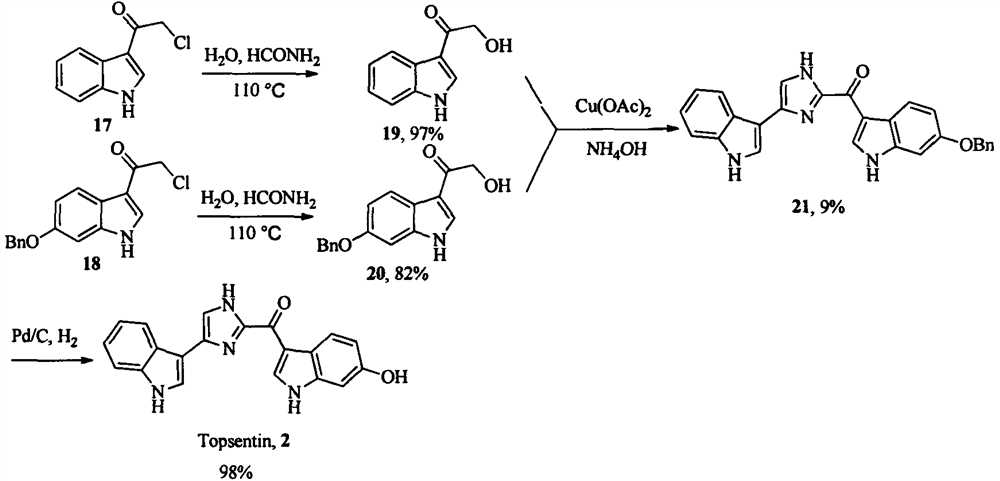
Topsentin derivatives and their preparation and application in anti-plant viruses and bacteria
A technology of derivatives and peanuts, applied in the direction of chemicals for biological control, botany equipment and methods, applications, etc., can solve the problems of low total yield, low natural content of alkaloids, and insufficient research on biological activity. Reaching the effect of good anti-plant virus and pathogenic bacteria activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1: Synthesis of Topsentin derivatives I-1 and I-7
[0076]
[0077] 61: Add (20mmol) indole and 80mL anhydrous ether into a 250mL round bottom flask, add 2.56mL (26mmol) of oxalyl chloride in ether solution dropwise at 0°C, react at 0°C for about 1.5h, monitor by TLC, the reaction is complete, Compound 60 was directly added 15 mL of absolute ethanol dropwise to the reaction solution without treatment, stirred at room temperature for 30 min, and monitored by TLC. After the reaction was completed, bright yellow powder 61 was obtained by suction filtration. The two-step yield was 86%, and the melting point was decomposed at 174°C. 1 H NMR (400MHz, DMSO-d 6 )δ12.48(s, 1H), 8.47(d, J=3.2Hz, 1H), 8.09(d, J=8.5Hz, 1H), 7.75(d, J=1.5Hz, 1H), 7.43(dd, J=8.5, 1.7Hz, 1H), 4.36(q, J=7.1Hz, 2H), 1.34(t, J=7.1Hz, 3H).
[0078] 62: In a 100mL round bottom flask, add compound 61 (5mmol), 40mL dichloromethane, add Et under stirring at 0°C 3 NO.9mL (1.4mmol) and 0.05g DM...
Embodiment 2
[0085] Embodiment 2: Synthesis of Topsentin derivatives I-2 and I-3
[0086]
[0087] I-2: Dissolve Topsentin A (0.16g, 0.5mmol) in 150mL dichloromethane, add Et 3 N (0.5mL, 3mmol), stirred at room temperature for about 10min, added Boc 2 O (0.44g, 2mmol), stirred at room temperature, monitored by TLC, after the reaction was complete, added water, extracted with dichloromethane, washed with saturated NaCl, anhydrous NaCl 2 SO 4 After drying and precipitating, column chromatography (PE:EA=15:1) separated to obtain 0.26 g of light yellow solid, yield 83%, melting point: 161-162°C. 1 H NMR (400MHz, CDCl 3 )δ8.59(s, 1H), 8.48-8.46(m, 1H), 8.26(d, J=7.7Hz, 1H), 8.19(d, J=7.2Hz, 1H), 8.05(s, 1H), 7.99(d, J=7.5Hz, 1H), 7.84(s, 1H), 7.45-7.42(m, 2H), 1.68(s, 18H), 1.53(s, 9H). 13 C NMR (100MHz, CDCl 3 )δ 179.7, 149.6, 149.0, 146.9, 145.4, 136.8, 135.9, 135.7, 135.1, 128.0, 127.7, 125.8, 124.7, 124.7, 123.6, 123.1, 122.6, 120.3, 119.0, 13.0, 115.8, 115.5 , 85.53, 84.0, 28.2, ...
Embodiment 3
[0089] Embodiment 3: Synthesis of Topsentin derivatives I-4, I-5 and I-6
[0090]
[0091] I-4: Dissolve Topsentin A (0.21g, 0.65mmol) in a mixed solution of 20mL dichloromethane and 20mL methanol, add camphorsulfonic acid (0.15g, 0.65mmol), heat to reflux for 2h, after precipitation, dichloromethane Methane was recrystallized, and 0.33 g of yellow crystals was obtained by suction filtration, yield 94.3%, melting point: 267-270°C. 1 H NMR (400MHz, DMSO-d 6 )δ12.65(s, 1H), 11.77(s, 1H), 8.76(s, 1H), 8.32(d, J=5.4Hz, 1H), 8.17(s, 2H), 8.03(d, J=7.5 Hz, 1H), 7.64(d, J=5.7Hz, 1H), 7.55(d, J=7.7Hz, 1H), 7.40-7.30(m, 2H), 7.29-7.10(m, 2H), 2.98(d , J=14.7Hz, 1H), 2.68(t, J=11.6Hz, 1H), 2.48(d, J=15.0Hz, 1H), 2.24(d, J=18.0Hz, 1H), 1.93(s, 1H ), 1.89-1.83(m, 1H), 1.80(d, J=18.1Hz, 1H), 1.42-1.20(m, 1H), 1.04(s, 3H), 0.74(s, 3H). 13 C NMR (100MHz, DMSO-d 6 )δ 216.6, 172.7, 141.8, 139.0, 137.3, 136.9, 126.4, 126.0, 124.7, 124.4, 123.3, 122.7, 121.8, 120.8, 119.8, 117.0, 114.0, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com