Uncaria amide A as well as pharmaceutical composition and application thereof
A technology of rhynchiferamide and composition, which is applied in the direction of drug combination, sugar derivatives, sugar derivatives, etc., and can solve the problems of no rhynchamide A pharmaceutical composition, no rhynchiferamide A, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
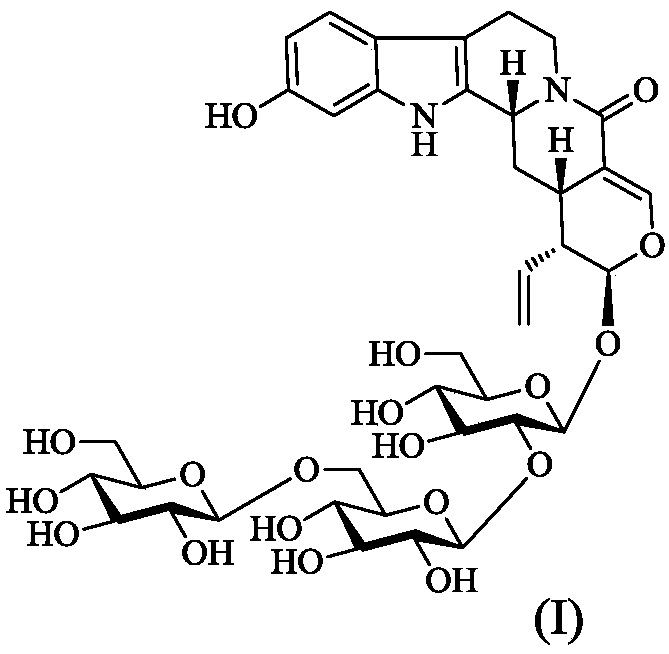
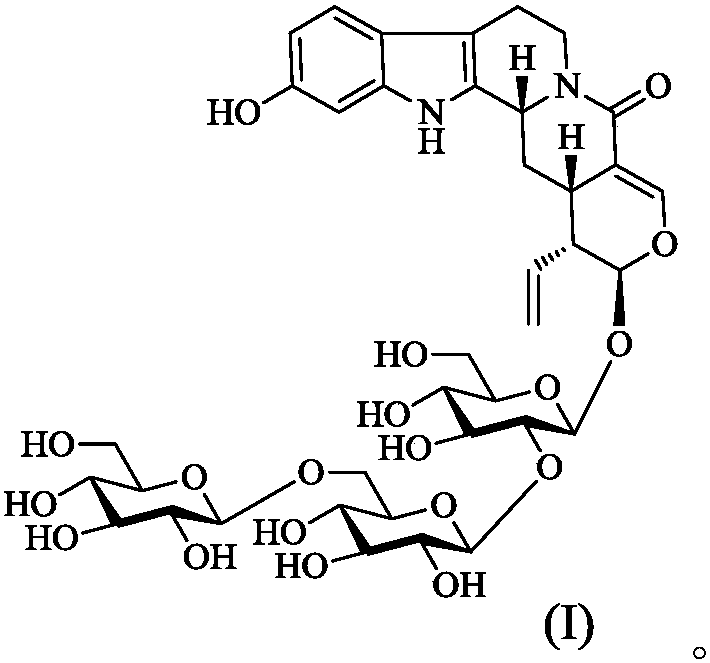
[0023] Preparation of compound 1:
[0024] Take the dried hooked stems and branches of Uncaria uncaria, crush them, extract twice with 70% ethanol under reflux, each time for 3 hours, combine the ethanol extracts, recover the ethanol under reduced pressure to obtain the extract. The extract was dissolved in 5 times the amount of water, and the D101 macroporous resin column chromatography was used for ethanol-water gradient elution. Combine the fractions eluted with 50% ethanol, concentrate, dissolve in methanol, adsorb on silica gel, place at room temperature to evaporate the solvent, grind and sieve, and perform silica gel column chromatography, elute with chloroform-methanol-water (7:3:0.3), Four fractions A-D were obtained. Fraction C was further prepared by medium-pressure MCI CHP-20P gel column, methanol-water gradient elution (1:9~9:1), and 5 fractions were obtained, C-1~C-5. Fraction C-2 was purified by HPLC using Rp-C 18 Column, acetonitrile-water gradient elution (...
Embodiment 2
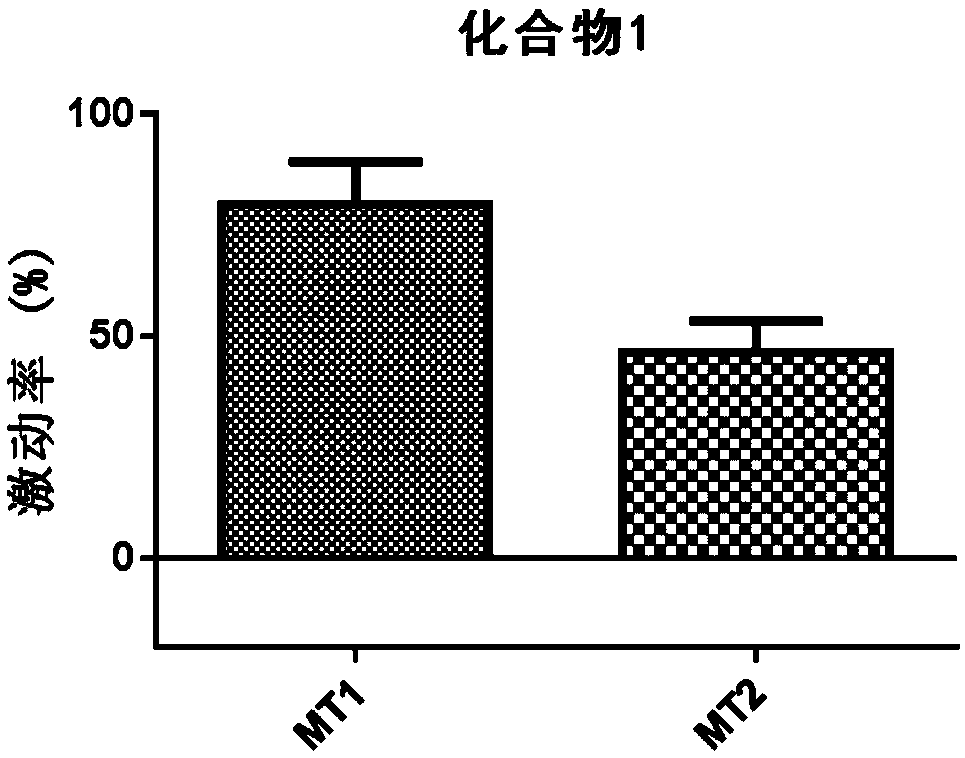
[0042] Compound 1 on melatonin receptor MT 1 and MT 2 Agonistic activity of the receptor.
[0043] 1 Materials and methods
[0044] 1.1 Materials:
[0045] melatonin receptor MT 1 and MT 2 The cell lines used for agonistic activity screening correspond to human kidney epithelial cells HEK293-MT 1 and HEK293-MT 2 ; Cell culture medium (Dulbecco's Modified Eagle Medium, DMEM) containing 10% fetal bovine serum; No-wash calcium flow kit.
[0046] 1.2 Instrument: CO 2 Constant temperature incubator Thermo Forma 3310 (USA); Inverted biological microscope XD-101 (Nanjing); Flexstation 3Benchtop Multi-Mode Microplate Reader (Molecular Devices, Sunnyvale, California, USA).
[0047] 1.3 Experimental process
[0048] Coat the 96-well black-walled transparent-bottom cell culture plate with the substrate BD Matrigel, put it in a constant temperature incubator at 37°C for 1 hour, absorb the supernatant, and dilute it with 4×10 4 Density per well, the corresponding HEK293 cells wer...
preparation Embodiment
[0055] 1. Compound 1 was prepared according to the method of Example 1. After dissolving with a small amount of DMSO, water for injection was added as usual, finely filtered, potted and sterilized to make an injection.
[0056] 2. Compound 1 was prepared according to the method of Example 1. After dissolving with a small amount of DMSO, it was dissolved in sterile water for injection, stirred to dissolve, filtered with a sterile suction filter funnel, then sterile fine filtered, and packed In the ampoule, freeze-dried at low temperature and sealed aseptically to obtain powder injection.
[0057] 3. According to the method of Example 1, the compound 1 was first prepared, and the excipient was added according to the ratio of its weight to the excipient at a ratio of 9:1 to make a powder.
[0058] 4. According to the method of Example 1, Compound 1 was firstly prepared, and the weight ratio of compound 1 to the excipient was 5:1, and the excipient was added, granulated and compre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com