Acidic copper chloride etching liquid in-situ electrolytic regeneration and copper recycling method
A technology of electrolysis regeneration and recovery method, which is applied in the direction of electrolysis process, electrolysis components, electrodes, etc. It can solve the problems of complicated technical process and operation, chlorine and hydrogen precipitation operation, and the need for centrifugation and dehydration, so as to increase the reaction area and avoid precipitation. Chlorine, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
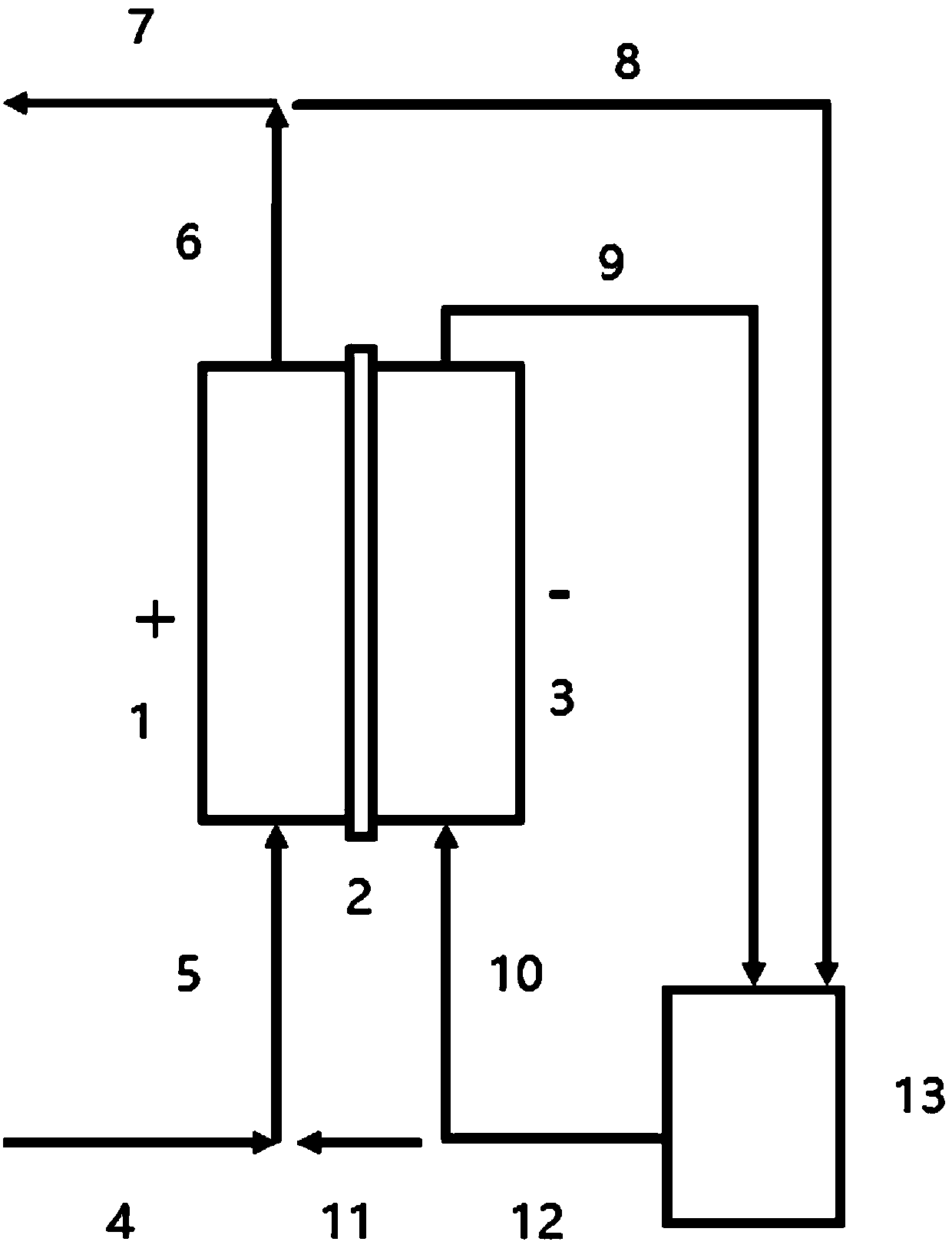
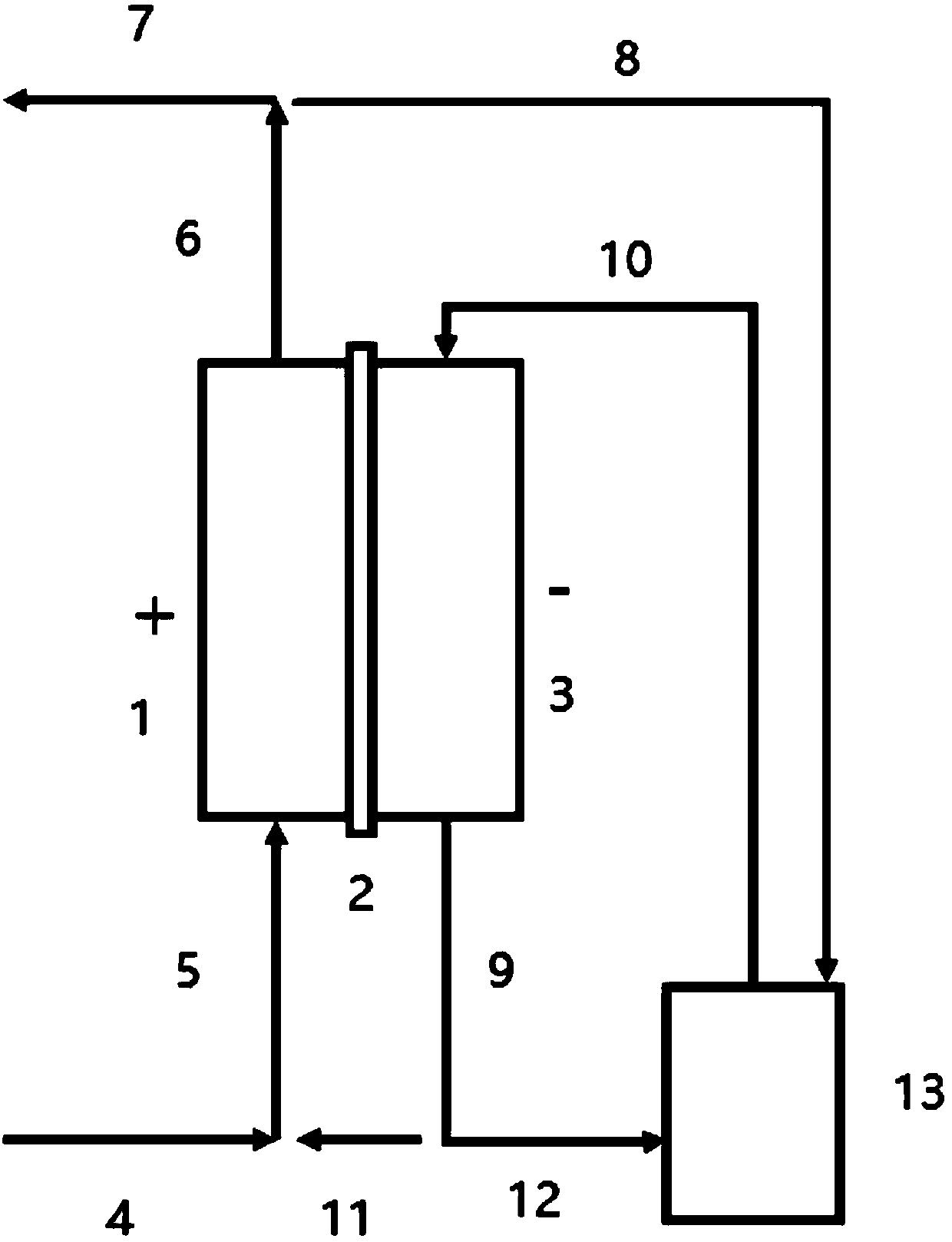
[0052] The composition of the initial cathodic solution is: CuCl 2 0.34mol / L, CuCl 0.16mol / L, NaCl 2mol / L, HCl 2mol / L. When the electrolytic regeneration and copper recovery system is in operation, the etching liquid stream 4 with a flow rate of 16.9 (Q) L / h and the catholyte branch stream 11 with a flow rate of 0.53 L / h (0.03 Q) are combined and used as the anode liquid stream 5 into the anode chamber 1 for regeneration. The anode outflow stream 6 is divided into a regenerated etchant stream 7 and a regenerated etchant branch 8 with a flow rate of 0.53L / h (0.03Q). The regenerated etching liquid branch stream 8 and the cathodic discharge stream 9 merge to form the cathode outdoor circulation stream 12, and the cathode outdoor circulation stream is divided into the catholyte branch stream 11 and the flow rate of 0.53L / h (0.03Q). The cathode inflow stream 10 of 3.96 L / h (0.23Q), the cathode inflow stream 10 enters the cathode chamber for copper deposition.
[0053] The appar...
Embodiment 2
[0055] The composition of the initial cathodic solution is: CuCl 2 0.50mol / L, CuCl 0.14mol / L, NaCl 2mol / L, HCl 2mol / L. When the electrolytic regeneration and copper recovery system is in operation, the etching liquid stream 4 with a flow rate of 17.0 (Q) L / h and the catholyte branch stream 11 with a flow rate of 0.60 L / h (0.035 Q) are merged and then used as the anode liquid stream 5 into the anode chamber 1 for regeneration. The anode outflow stream 6 is divided into a regenerated etchant stream 7 and a regenerated etchant branch 8 with a flow rate of 0.60L / h (0.035Q). The regenerated etching liquid branch stream 8 and the cathodic discharge stream 9 merge to form the cathode outdoor circulation stream 12, and the cathode outdoor circulation stream is divided into the catholyte branch stream 11 and the flow rate of 0.60L / h (0.035Q). 6.48L / h (0.38Q) of the cathode inflow stream 10, the cathode inflow stream 10 enters the cathode chamber for copper deposition.
[0056] The ...
Embodiment 3
[0058] The composition of the initial cathodic solution is: CuCl 2 0.40mol / L, CuCl 0.15mol / L, NaCl 2mol / L, HCl 2mol / L. When the electrolytic regeneration and copper recovery system is in operation, the etching liquid stream 4 with a flow rate of 20.1 (Q) L / h and the catholyte branch stream 11 with a flow rate of 0.67 L / h (0.033 Q) are combined and used as the anode liquid stream 5 into the anode chamber 1 for regeneration. The anode outflow stream 6 is divided into a regenerated etchant stream 7 and a regenerated etchant branch 8 with a flow rate of 0.67L / h (0.033Q). The regenerated etching liquid branch stream 8 and the cathodic discharge stream 9 merge to form the cathode outdoor circulation stream 12, and the cathode outdoor circulation stream is divided into the catholyte branch stream 11 and the flow rate of 0.67L / h (0.033Q). The cathode inflow stream 10 of 9.36L / h (0.47Q), the cathode inflow stream 10 enters the cathode chamber for copper deposition.
[0059] The app...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com