A kind of synthetic method and application of helical indole compound based on sarcosine and polycarbonyl cyclic ketone compound
A synthesis method and compound technology, which can be applied in the fields of organic chemistry, drug combination, antitumor drugs, etc., can solve the problems of high price of reaction raw materials, difficult operation, complicated synthesis steps, etc., and achieve environmental protection and pollution-free reaction solvent, convenient operation, and raw materials. Inexpensive and accessible effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A kind of synthetic method of helical indole compound based on sarcosine, described synthetic method comprises the following steps:
[0042] (1) Weigh 1mmol 2-vinylpyridylindoline, 1mmol sarcosine and 1mmol isatin respectively, place them in 50mL of ethanol aqueous solution reaction solvent, and heat, stir and reflux for 3 hours under air environment;
[0043] (2) After the reaction finishes, wait until the temperature is cooled to room temperature, and the reaction product is suction filtered to obtain a solid crude product, and then the solid crude product is filtered with CH 3 OH / DMF mixed solvent was used for recrystallization, and after recrystallization twice, a white solid, pyridyl indoline compound 1 was obtained; the yield was 73%, and the melting point was 102-104°C.
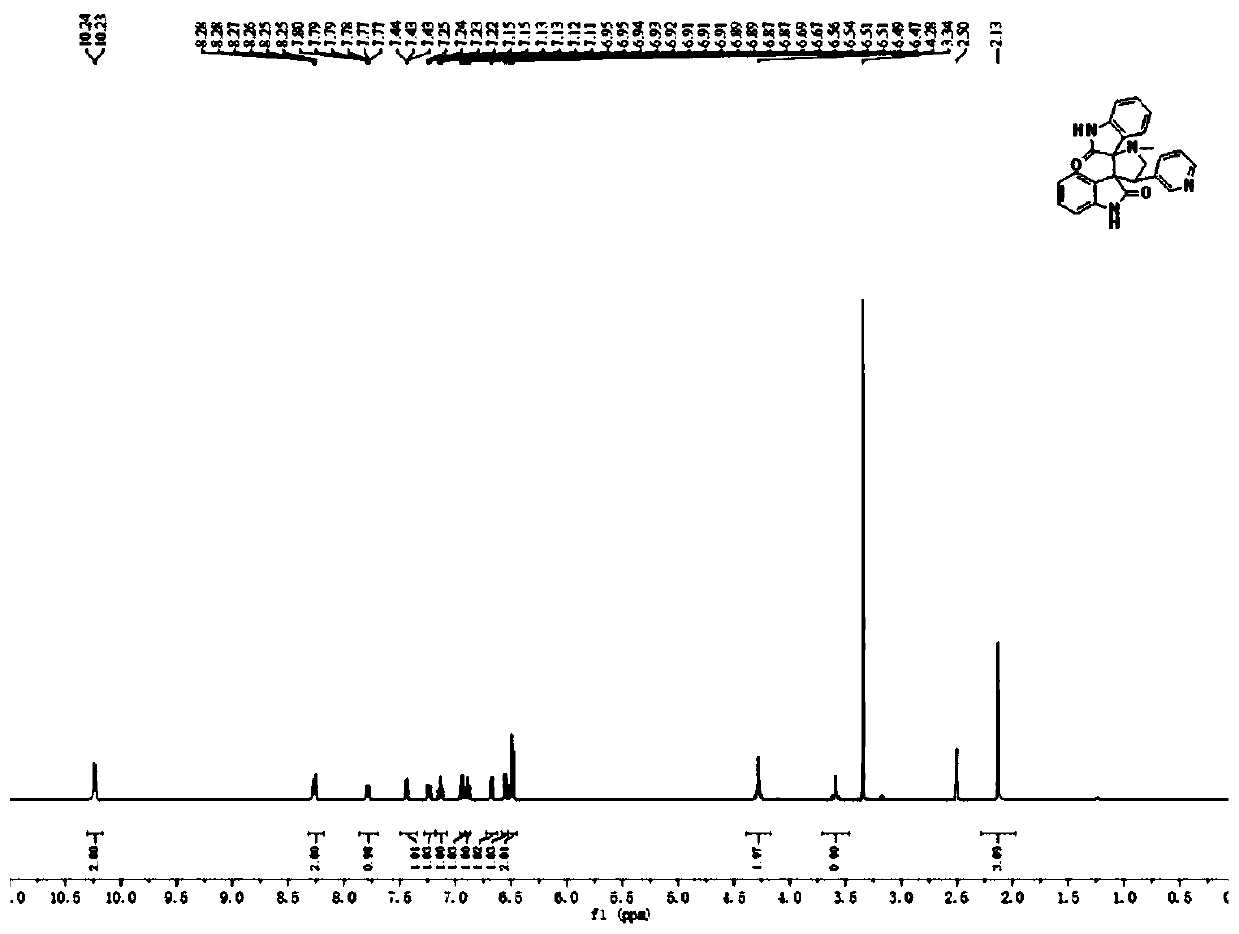
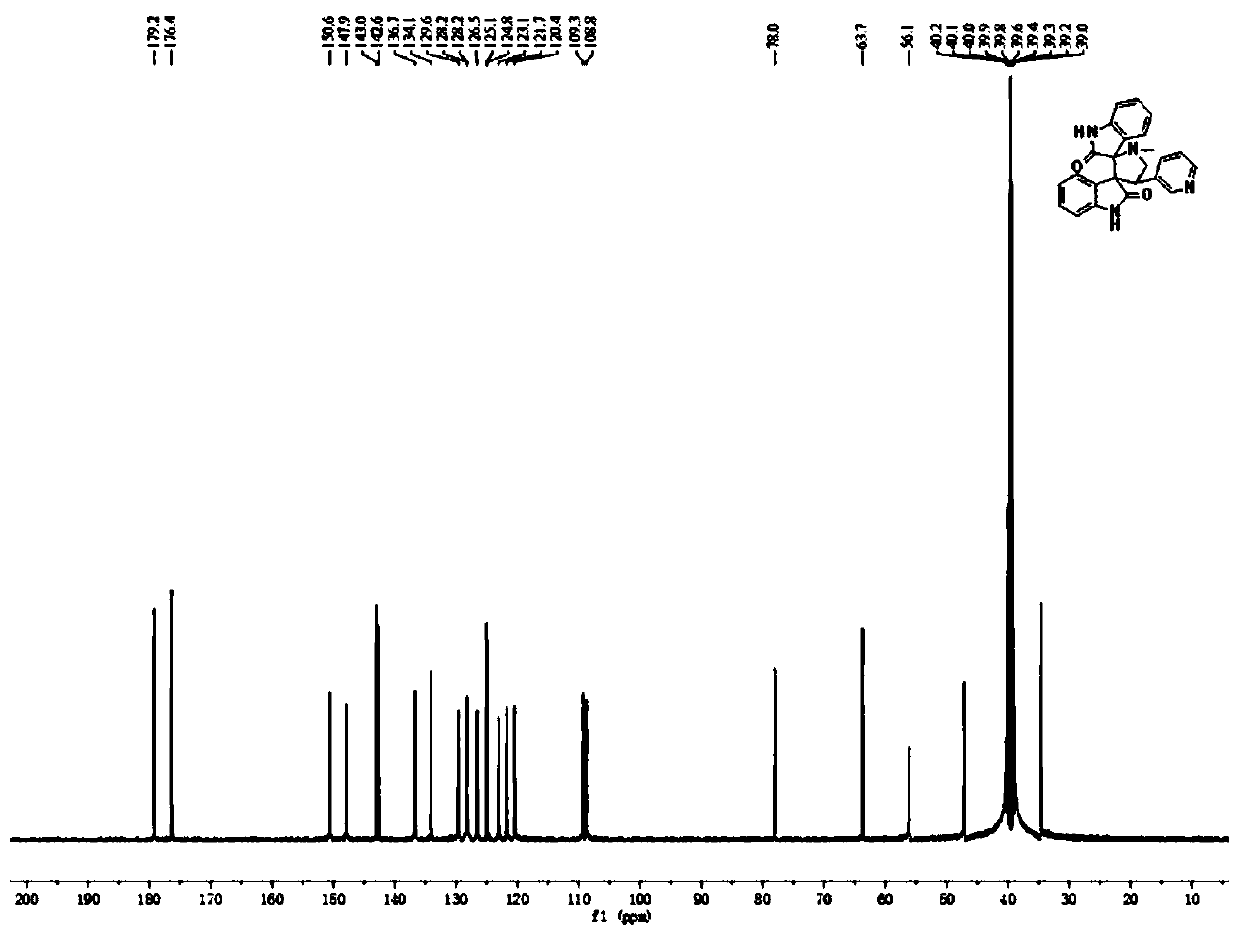
[0044] The product structural formula of gained pyridyl indoline compound 1 is:
[0045]
[0046] H NMR spectrum data of the product:
[0047] 1 H NMR (400MHz, DMSO-d6 )δ10.23(d,J=5.8Hz,2H...
Embodiment 2
[0051] A kind of synthetic method of helical indole compound based on sarcosine, described synthetic method comprises the following steps:
[0052] (1) Weigh 1mmol 2-vinylpyridylindoline, 1mmol sarcosine and 1mmol acenaphthenequinone respectively, place them in 50mL ethanol aqueous solution reaction solvent, and heat, stir and reflux for 2 hours under air environment;
[0053] (2) After the reaction finishes, wait until the temperature is cooled to room temperature, and the reaction product is suction filtered to obtain a solid crude product, and then the solid crude product is filtered with CH 3 OH / DMF mixed solvent was used for recrystallization, and after recrystallization twice, an orange solid, that is, pyridyl indoline compound 2 was obtained; the yield was 85%, and the melting point was 77-79°C.
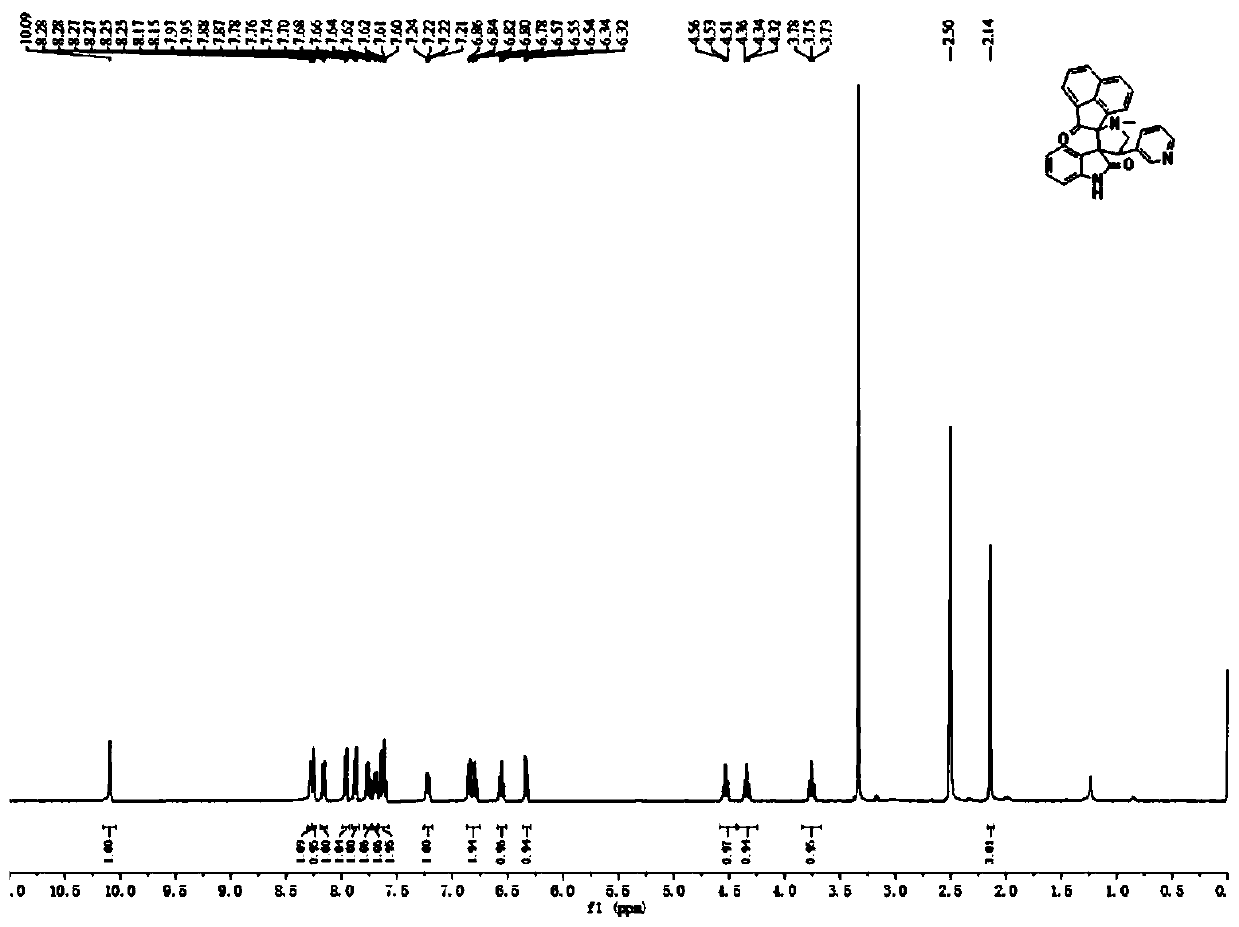
[0054] The product structural formula of gained pyridyl indoline compound 2 is:
[0055]
[0056] H NMR spectrum data of the product:
[0057] 1 H NMR (400MHz, DMSO-d 6...
Embodiment 3
[0061] A kind of synthetic method of helical indole compound based on sarcosine, described synthetic method comprises the following steps:
[0062] (1) Weigh 1mmol 2-vinylpyridylindoline, 1mmol sarcosine and 1mmol ninhydrin respectively, place them in 50mL ethanol aqueous solution reaction solvent, and heat, stir and reflux for 3 hours under air environment;
[0063] (2) After the reaction finishes, wait until the temperature is cooled to room temperature, and the reaction product is suction filtered to obtain a solid crude product, and then the solid crude product is filtered with CH 3 OH / DMF mixed solvent was used for recrystallization, and after recrystallization twice, a yellow solid, that is, pyridylindoline compound 3 was obtained; the yield was 64%, and the melting point was 98-100°C.
[0064] The product structural formula of gained pyridyl indoline compound 3 is:
[0065]
[0066] H NMR spectrum data of the product:
[0067] 1 H NMR (400MHz, DMSO-d 6 )δ10.27(s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com