A method for detecting chiral isomers of key intermediates of avibactam sodium
A technology for avibactam sodium and chiral isomers, which is applied in the field of medicine and achieves the effects of strong practicability, convenient parameter adjustment and symmetrical peak shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
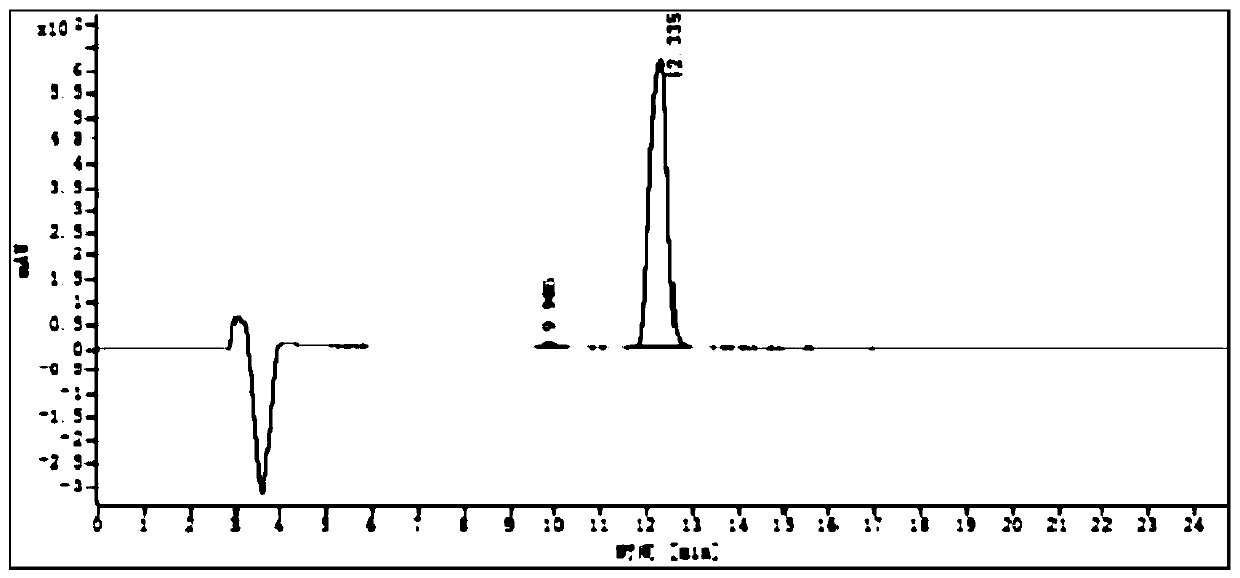
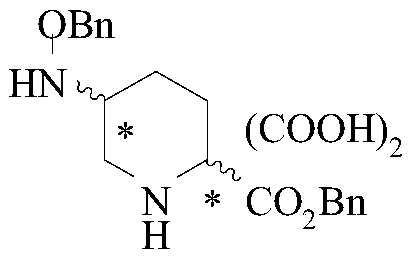
[0052] A sample of (2S,5R)-5-(benzyloxyamino)-piperidine-2-carboxylic acid benzyl oxalate whose batch number is 150804 is selected to detect the content of its chiral isomers, including the following steps:
[0053] S1, prepare the test solution:
[0054] Weigh 20mg of (2S,5R)-5-(benzyloxyamino)-piperidine-2-carboxylate benzyl oxalate sample, add 10mL of methanol, mix well, and prepare each 1mL containing (2S,5R)-5 -(Benzyloxyamino)-piperidine-2-carboxylic acid benzyl oxalate 2.0mg solution, as the test solution, stand-by.
[0055] S2. Prepare mixed control solution:
[0056] Weigh 2g (2S,5R)-5-(benzyloxyamino)-piperidine-2-carboxylic acid benzyl oxalate, 5mg (2S,5S)-5-(benzyloxyamino)-piperidine-2-carboxylic acid Benzyl ester oxalate, 5 mg (2R,5S)-5-(benzyloxyamino)-piperidine-2-carboxylic acid benzyl oxalate, and 5 mg (2R,5R)-5-(benzyloxyamino)-piperidine -2-benzyloxyamino)-piperidine-2-carboxylic acid benzyl oxalate, add 1000mL of methanol, mix well, and prepare each 1mL...
Embodiment 2
[0066] Choose the (2S,5R)-5-(benzyloxyamino)-piperidine-2-formic acid benzyl oxalate sample of the same batch as in Example 1, and detect the content of its chiral isomers. This implementation The difference between Example and Example 1 is only: the parameters of flow velocity in the chromatographic conditions are different, and other detection conditions are consistent with Example 1. In this embodiment, the assay conditions of high performance liquid chromatography include:
[0067] Chromatographic column: CHIRALPAK AD-H (model: length 250mm, inner diameter 4.6mm, amylose filler, filler particle size 5μm);
[0068] Detector: UV detector;
[0069] Detection wavelength: 280nm;
[0070] Column temperature: 30°C;
[0071] Flow rate: 0.8mL / min;
[0072] Mobile phase: calculated by volume ratio, n-hexane: isoamyl alcohol: ethanol: diethylamine=850:150:50:0.5.
[0073] Calculated by external standard method, in (2S,5S)-5-(benzyloxyamino)-piperidine-2-carboxylic acid benzyl ox...
Embodiment 3
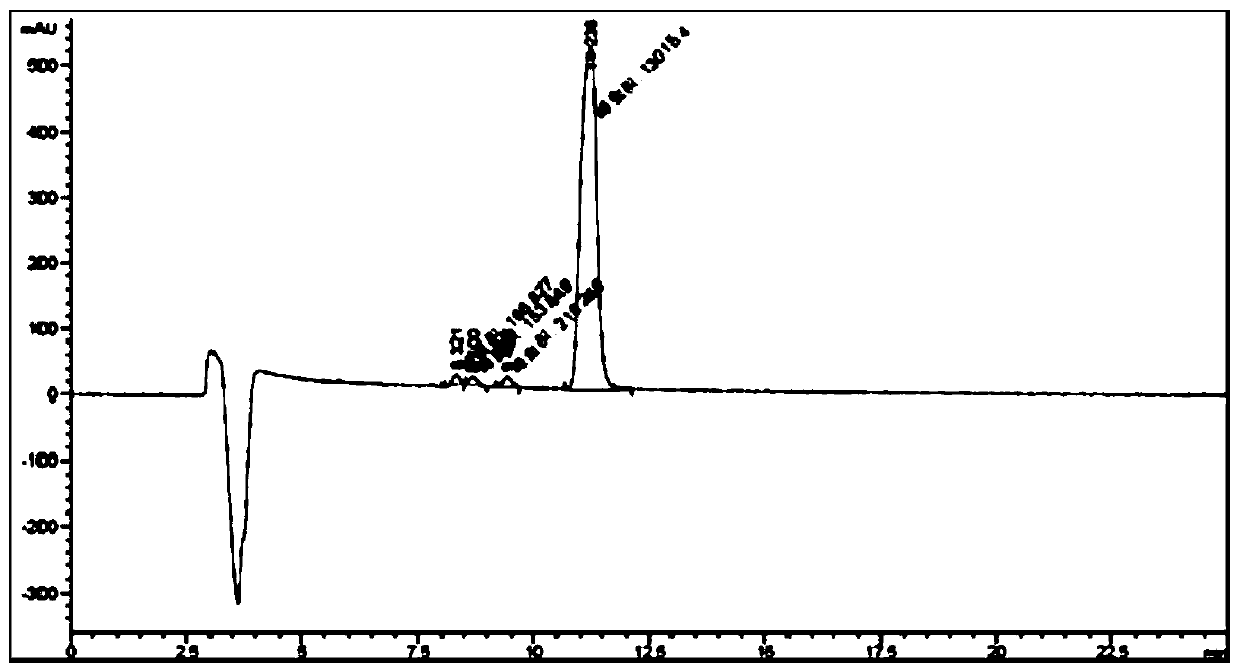
[0075] Choose the (2S,5R)-5-(benzyloxyamino)-piperidine-2-formic acid benzyl oxalate sample of the same batch as in Example 1, and detect the content of its chiral isomers. This implementation The difference between Example and Example 1 is only: the parameters of flow velocity in the chromatographic conditions are different, and other detection conditions are consistent with Example 1. In this embodiment, the assay conditions of high performance liquid chromatography include:
[0076] Chromatographic column: CHIRALPAK AD-H (model: length 250mm, inner diameter 4.6mm, amylose filler, filler particle size 5μm);
[0077] Detector: UV detector;
[0078] Detection wavelength: 280nm;
[0079] Column temperature: 30°C;
[0080] Flow rate: 1.2mL / min;
[0081] Mobile phase: calculated by volume ratio, n-hexane: isoamyl alcohol: ethanol: diethylamine=850:150:50:0.5.
[0082]Calculated by external standard method, in (2S,5S)-5-(benzyloxyamino)-piperidine-2-carboxylic acid benzyl oxa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com