A high-concentration nimotuzumab preparation for subcutaneous or intramuscular injection and its preparation method and application
A Nimotuzumab and intramuscular injection technology, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, antibodies, etc., to achieve the effect of system stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] In this example, the formula of the Nimotuzumab preparation is as follows: 30 mg / mL Nimotuzumab, 10 mM phosphate buffer (pH7.0), 60 mM trehalose dihydrate, 0.02% polysorbate 20, 1000IU / mL hyaluronidase.
[0042] The preparation method is as follows:
[0043] (1) Nimotuzumab is added to the buffer solution of the buffer, a nonionic surfactant is added thereto, and then hollow fiber ultrafiltration is performed to obtain a buffer system of Nimotuzumab;
[0044] (2) Add hyaluronidase and a stabilizer to the buffer system of Nimotuzumab obtained in step (1), and mix to obtain the Nimotuzumab preparation.
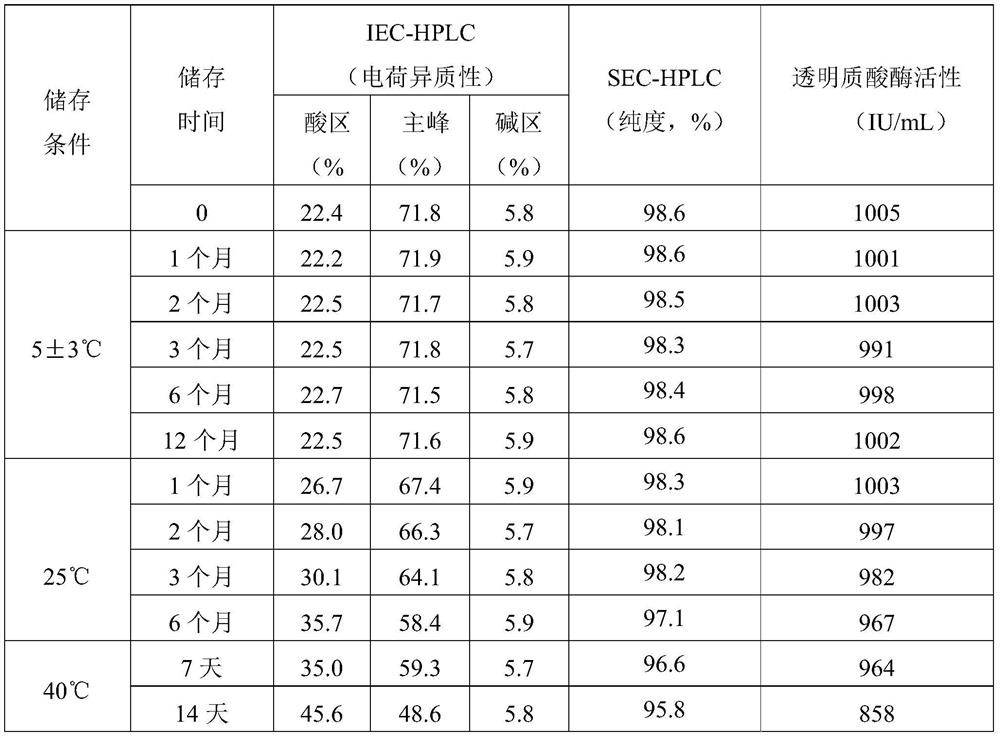
[0045] The stability data of the nimotuzumab preparations prepared in this example are shown in Table 1.
[0046] Formulation stability data described in table 1 embodiment 1
[0047]
[0048] As can be seen from Table 1, the preparation formula described in Example 1 is stable under storage conditions (2-8° C.).
Embodiment 2
[0050] In this embodiment, the formulation of the Nimotuzumab preparation is as follows: 50 mg / mL Nimotuzumab, 15 mM phosphate buffer (pH6.8), 80 mM trehalose dihydrate, 0.04% polysorbate 20 , 1500IU / mL hyaluronidase.
[0051] The preparation method is as follows:
[0052] (1) Nimotuzumab is added to the buffer solution of the buffer, a nonionic surfactant is added thereto, and then hollow fiber ultrafiltration is performed to obtain a buffer system of Nimotuzumab;
[0053] (2) Add hyaluronidase and a stabilizer to the buffer system of Nimotuzumab obtained in step (1), and mix to obtain the Nimotuzumab preparation.
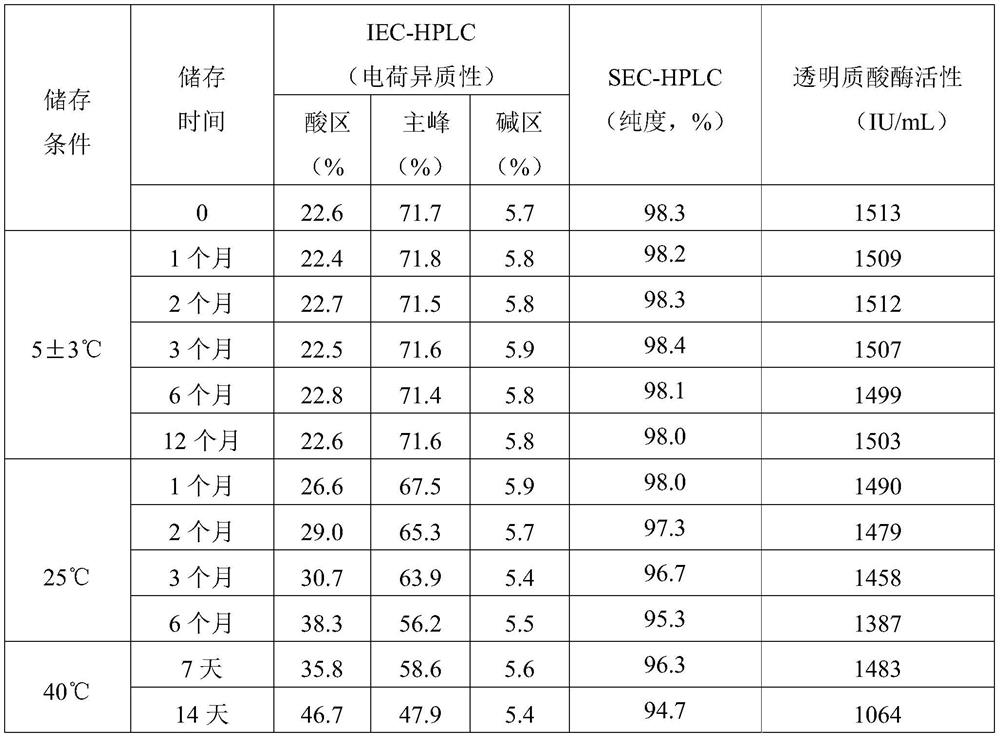
[0054] The stability data of the nimotuzumab preparations prepared in this example are shown in Table 2.
[0055] Formulation stability data described in table 2 embodiment 2
[0056]
[0057] As can be seen from Table 2, the formula described in Example 2 is stable under its storage conditions (2-8° C.).
Embodiment 3
[0059] In this embodiment, the formulation of the Nimotuzumab preparation is as follows: 100 mg / mL Nimotuzumab, 20 mM phosphate buffer (pH7.0), 100 mM trehalose dihydrate, 0.05% polysorbate 20 , 1500IU / mL hyaluronidase.
[0060] The preparation method is as follows:
[0061] (1) Nimotuzumab is added to the buffer solution of the buffer, a nonionic surfactant is added thereto, and then hollow fiber ultrafiltration is performed to obtain a buffer system of Nimotuzumab;
[0062] (2) Add hyaluronidase and a stabilizer to the buffer system of Nimotuzumab obtained in step (1), and mix to obtain the Nimotuzumab preparation.
[0063] The stability data of the Nimotuzumab preparation prepared by this embodiment are shown in Table 3
[0064] Formulation stability data described in table 3 embodiment 3
[0065]
[0066]
[0067] As can be seen from Table 3, the formula described in Example 3 is stable under its storage conditions (2-8° C.).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com