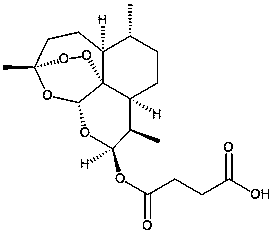
Artesunate preparation for injection, and application thereof
A technology for artesunate and injection, which is applied in the field of drug preparation, can solve the problems of time-consuming dissolution, good stability, complexity and the like, and achieves the effects of simplified production and packaging process, less local irritation, and suitable for injection use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
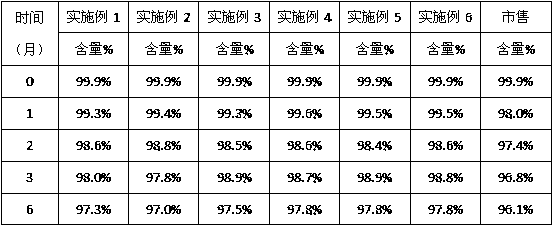
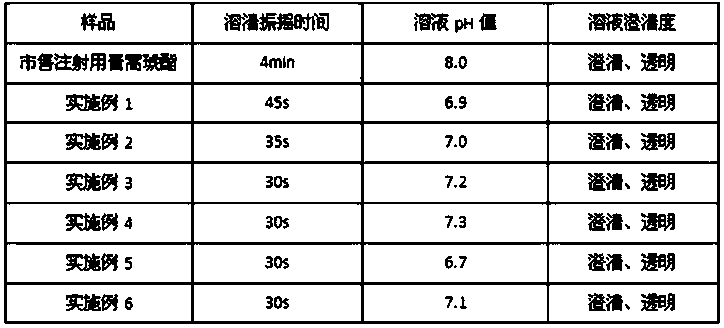
Embodiment 1
[0029] Prescription: Artesunate 1000 parts, disodium hydrogen phosphate 554 parts (weight)
[0030] Preparation method: take sterile artesunate for injection and disodium hydrogen phosphate for injection out of the outer packaging, remove dust and clean, wipe and sterilize, and transfer them to a sterile room for standby; After the disodium hydrogen phosphate is separately pulverized, 100-mesh sieve is used; the sieved artesunate and disodium hydrogen phosphate are mixed evenly according to the prescription ratio, and after passing the full inspection, they are divided into vials according to the clinically required dose, and the stoppers are pressed. , capping, light inspection, qualified inspection, labeling, and packaging to obtain artesunate for injection.
Embodiment 2
[0032] Prescription: 1000 parts of artesunate, 738 parts of disodium hydrogen phosphate (by weight)
[0033] Preparation method: take sterile artesunate for injection and disodium hydrogen phosphate for injection out of the outer packaging, remove dust and clean, wipe and sterilize, and transfer them to a sterile room for standby; After the disodium hydrogen phosphate is separately pulverized, 100-mesh sieve is used; the sieved artesunate and disodium hydrogen phosphate are mixed evenly according to the prescription ratio, and after passing the full inspection, they are divided into vials according to the clinically required dose, and the stoppers are pressed. , capping, light inspection, qualified inspection, labeling, and packaging to obtain artesunate for injection.
Embodiment 3
[0035] Prescription: Artesunate 1000 parts, disodium hydrogen phosphate 1477 parts (weight)
[0036]Preparation method: take sterile artesunate for injection and disodium hydrogen phosphate for injection out of the outer packaging, remove dust and clean, wipe and sterilize, and transfer them to a sterile room for standby; After the disodium hydrogen phosphate is separately pulverized, 100-mesh sieve is used; the sieved artesunate and disodium hydrogen phosphate are mixed evenly according to the prescription ratio, and after passing the full inspection, they are divided into vials according to the clinically required dose, and the stoppers are pressed. , capping, light inspection, qualified inspection, labeling, and packaging to obtain artesunate for injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com