Freeze-drying technology for cardiomyopeptidin for injection
A technology for injection and cardiac peptide, which is applied in the field of freeze-drying process of cardiac peptide for injection, which can solve the problems of unclear improvement of heart function, influence on purity and activity, and poor appearance of freeze-dried products, so as to save freeze-dried Drying time, improved industrial production efficiency, and low water content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
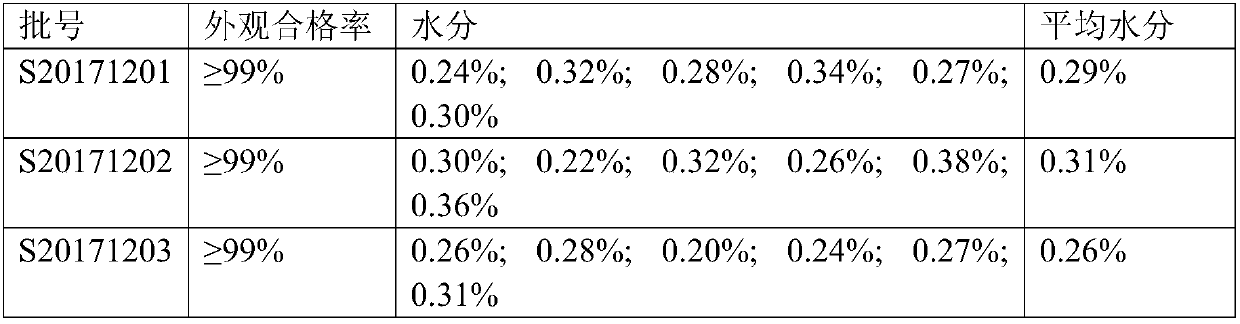
Embodiment 1
[0018] (1) 3% volume fraction tert-butanol is added in the pretreatment stage of the cardiac peptide solution, and the processed cardiac peptide solution is sub-packed in 10ml low borosilicate glass control injection bottles, and the specification is 20mg / bottle (calculated by polypeptide), vials The inner solution is about 4ml.
[0019] (2) Put the aliquoted cardiopeptide into a freeze-drying chamber, rapidly lower the temperature in the freeze-drying chamber to -18.0° C., and keep it for 1 hour.
[0020] (3) Rapidly lower the temperature of the freeze-drying chamber to -50.0° C. and keep it for 6 hours.
[0021] (4) Adjust the temperature of the condenser to -50.0°C, pre-evacuate the system to 40Pa, and start the primary drying. Control the temperature and vacuum degree, the temperature rises from -50.0°C to 0.0°C within 20h, and maintains at 0.0°C for 2h. The vacuum degree was reduced from 40Pa to 20Pa within 20h.
[0022] (5) Enter the desorption drying stage, control t...
Embodiment 2
[0025] (1) 5% volume fraction tert-butanol is added in the pretreatment stage of the cardiac peptide solution, and the processed cardiac peptide solution is subpackaged in 10ml low-borosilicate glass control injection bottles, and the specification is 20mg / bottle (calculated by polypeptide), vials The inner solution is about 4ml.
[0026] (2) Put the aliquoted cardiopeptide into the freeze-drying chamber, rapidly lower the temperature in the freeze-drying chamber to -18.0°C, and keep it for 1 hour.
[0027] (3) Rapidly lower the temperature of the freeze-drying chamber to -50.0°C and keep it for 6 hours.
[0028] (4) Adjust the temperature of the condenser to -50.0°C, pre-evacuate the system to 40Pa, and start the primary drying. Control the temperature and vacuum degree, the temperature is raised from -50.0°C to 0.0°C in 20h, and kept at 0.0°C for 1h. 20h vacuum from 40Pa to 20Pa.
[0029] (5) Enter the analytical drying stage, control the temperature and vacuum degree, th...
Embodiment 3
[0032] (1) Add 7% volume fraction tert-butanol in the pretreatment stage of the cardiopeptide solution, and the processed cardiopeptide solution is divided into 10ml low-borosilicate glass control injection bottles, and the specification is 20mg / bottle (calculated by polypeptide), vials The inner solution is about 4ml.
[0033] (2) Put the aliquoted cardiopeptide into the freeze-drying chamber, rapidly lower the temperature in the freeze-drying chamber to -18.0°C, and keep it for 1 hour.
[0034] (3) Rapidly lower the temperature of the freeze-drying chamber to -50.0°C and keep it for 5 hours.
[0035] (4) Adjust the temperature of the condenser to -50.0°C, pre-evacuate the system to 40Pa, and start the primary drying. Control the temperature and vacuum degree, the temperature is raised from -50.0°C to 0.0°C in 20h, and kept at 0.0°C for 1h. 20h vacuum from 40Pa to 20Pa.
[0036] (5) Enter the analytical drying stage, control the temperature and vacuum degree, the temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com