Organic chemical synthesis reactor capable of being integrally operated
A synthetic reactor and organic chemistry technology, applied in chemical/physical/physical chemical fixed reactors, chemical/physical/physical chemical nozzle reactors, chemical instruments and methods, etc., can solve the complicated operation process, energy and Problems such as waste of test time and lack of light source, etc., achieve the effect of simple and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1 Synthesis of sulfonamide compounds based on arylpyrazole skeleton
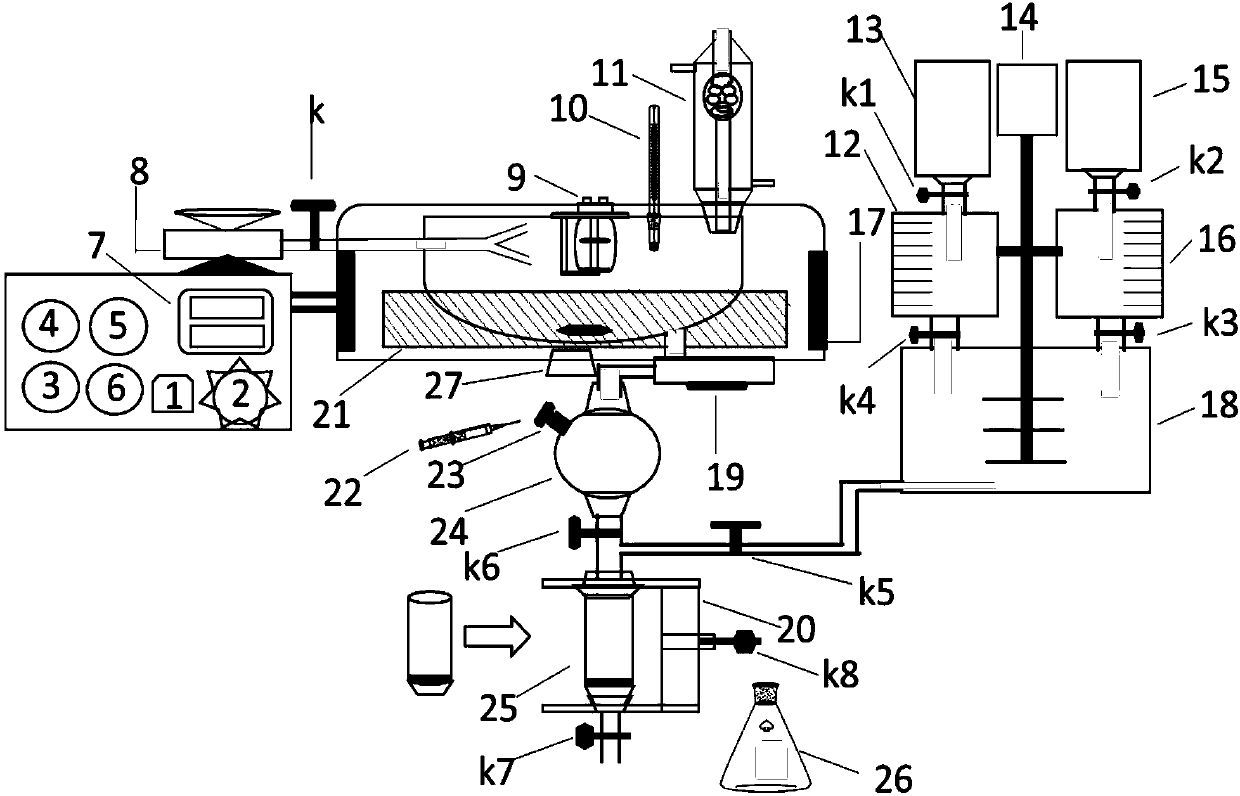
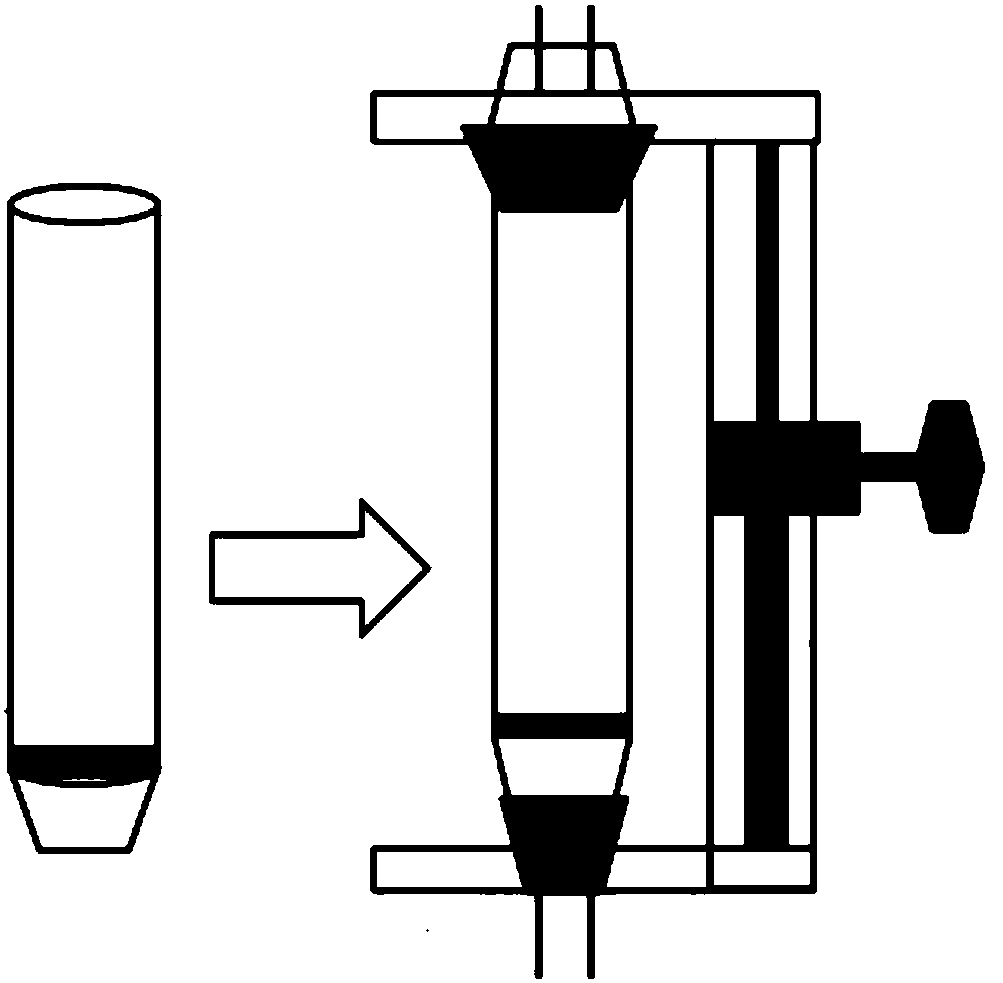
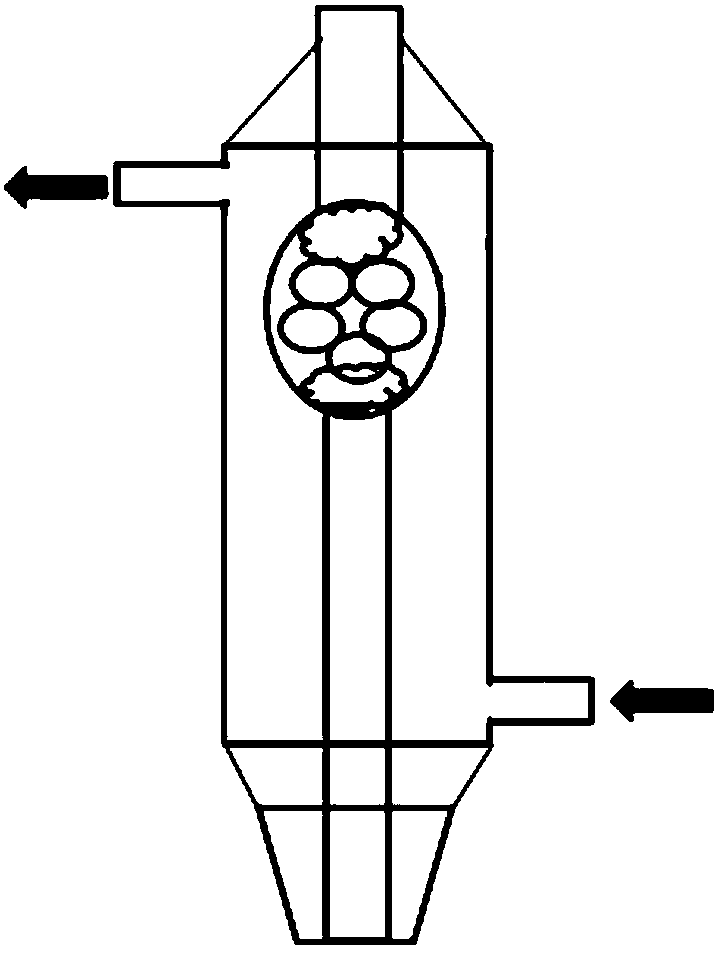
[0052] Take a dry 50mL beaker, add 4.3g (0.01mol) fipronil to the beaker, measure 15mL of ethyl acetate with a graduated cylinder and pour it into the beaker, then weigh 0.48g60% NaH (0.02mol) with weighing paper and slowly Add it into the beaker and keep stirring to make it fully dissolved, then add 1.9g (0.01mol) p-benzenesulfonyl chloride and continue stirring to make it evenly mixed. After the reaction raw materials are processed, press the xenon lamp power button 1 and adjust the xenon lamp brightness adjustment knob 2 to let the xenon lamp 9 emit light for test pretreatment, then press the oil bath power button 3 to use the oil bath 21 for heating, and the magnetic stirrer 27 also starts Rotate for stirring, then transfer the reaction raw materials to the funnel on the upper part of the ultrasonic nebulizer 8, and at the same time turn on the rotary switch k to use the ultrasonic nebu...
Embodiment 2
[0053] Conversion and separation of actinic isomers of embodiment 2 azobenzene
[0054] Take 4g of anti-azobenzene and dissolve in 30mL of anhydrous benzene. After the anti-azobenzene is fully dissolved in anhydrous benzene, press the xenon lamp power button 1 and adjust the xenon lamp brightness adjustment knob 2 to let the xenon lamp 9 emit light. Carry out test pretreatment, then press the oil bath pot power button 3 to use the oil bath pot 21 to heat, and at the same time the magnetic stirrer 27 also starts to rotate for stirring work, and then transfer the reaction raw materials to the funnel on the upper part of the ultrasonic sprayer 8 while twisting and rotating Switch k uses the ultrasonic nebulizer 8 to carry out ultrasonic spray sample injection, and then presses the microwave switch button 4 to allow the microwave generator 17 to generate 400W microwave radiation to assist in the completion of the configuration conversion process. The temperature of the photoreactio...
Embodiment 3
[0055] Preparation and separation of embodiment 3 acetophenone
[0056]Take a 50mL dry beaker, weigh 2g of finely ground anhydrous aluminum trichloride, add 10mL of anhydrous benzene, slowly add 2mL of acetic anhydride during the stirring process, press the xenon lamp power button 1 after the raw materials are processed And adjust the xenon lamp brightness adjustment knob 2 to allow the xenon lamp 9 to emit light for test pretreatment, then press the oil bath power button 3 to use the oil bath 21 to heat while the magnetic stirrer 27 also starts to rotate for stirring, and then the reaction raw materials are transferred to Turn on the rotary switch k in the funnel on the upper part of the ultrasonic nebulizer 8 and utilize the ultrasonic nebulizer 8 to carry out ultrasonic spray sample injection, then press the microwave switch button 4 to allow the microwave generator 17 to produce 400W microwave radiation to participate in the auxiliary synthesis reaction, and the temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com