Gel factor capable of recognizing aromatic acid isomer and preparation and application of supramolecular polymer gel of aromatic acid isomer
A technology of supramolecular polymer and gel factor, applied in the field of gel factor and its synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, the preparation of gelling factor BP5
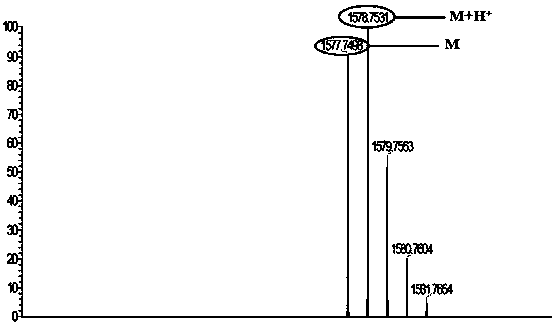
[0031] In 50ml of acetonitrile, add 0.58g (5×10 -3 mol) brominated column[5]arene P5, 0.43g (1.5×10 -3 mol) naphthalimide derivatives, reacted at 80°C for 48 hours, cooled to room temperature after the reaction was completed, removed the solvent by rotary evaporation, mixed the sample and put it on the column, with petroleum ether: ethyl acetate = 10:1~5 ( v / v) was eluted to obtain a yellow solid product which was the gelling factor BP5; the yield was 75.6%. Its mass spectrum see figure 1 . Its structural formula is as follows:
[0032] .
Embodiment 2
[0033] Embodiment 2, preparation of supramolecular polymer gel BP5G
[0034] Take 0.005 g of the sensor molecule prepared in Example 1, add it to 1 ml of cyclohexanol and heat to dissolve it fully, let it stand, and after cooling to room temperature, a supramolecular polymer gel BP5G with a mass volume ratio of 5 mg / ml is formed.
Embodiment 3
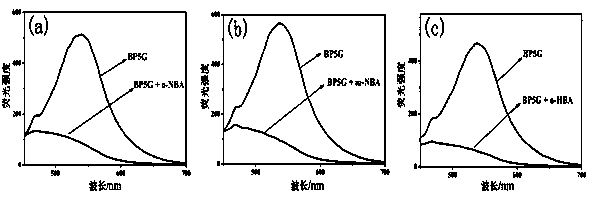
[0035] Embodiment 3, BP5G colorimetry / fluorescence recognition p-nitrobenzoic acid
[0036] Pipette the supramolecular polymer gel BP5G into three cuvettes respectively, add o-nitrobenzoic acid, m-nitrobenzoic acid and p-nitrobenzoic acid aqueous solution (concentration: 0.1M), if supramolecular polymerization The fluorescence of the gel BP5G was quenched, and the color of the gel changed from yellow to colorless, indicating that o-nitrobenzoic acid or m-nitrobenzoic acid was added dropwise; if the fluorescence of the gel BP5G did not quench, then It shows that p-nitrobenzoic acid is added dropwise.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com