Dihydromyricetin eye drop with synergistic effect and high aqueous solution stability and preparation method thereof
A technology of dihydromyricetin and high aqueous solution, which is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve problems such as strong irritation, improve stability and improve curative effect. , the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
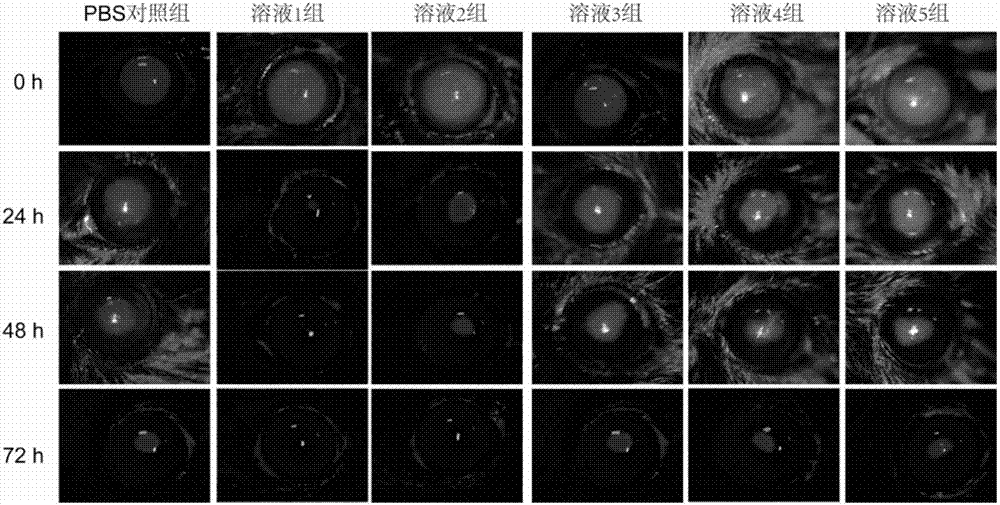
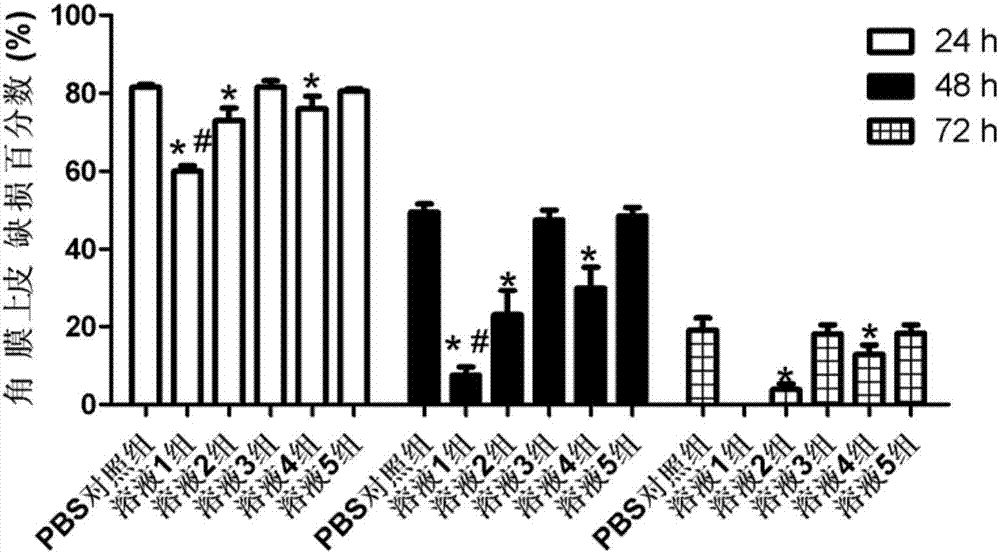
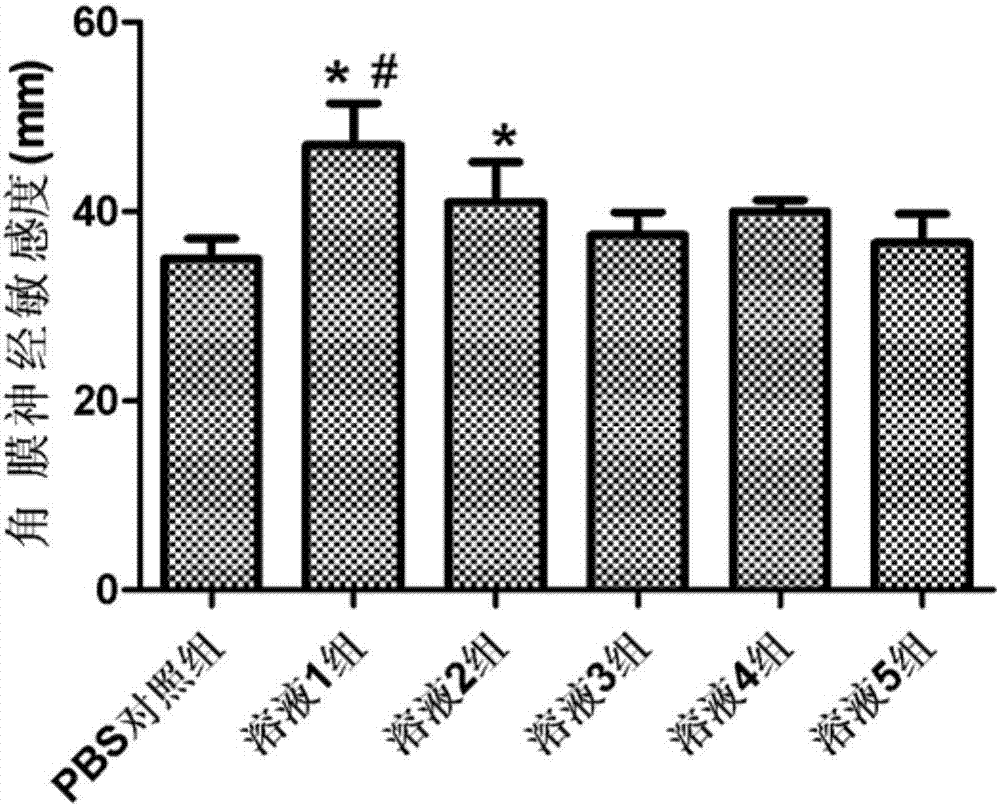
[0024] Put 50mg of dihydromyricetin and 900mg of rebaudioside A in a 100mL round-bottomed flask, add 50mL of absolute ethanol, after fully dissolving, evaporate the absolute ethanol in a 40°C water bath in a rotating vacuum to obtain a uniformly dispersed film of the drug and the copolymer , then add distilled water, fully wash the membrane, 300w power water bath ultrasonic 10min, get micellar solution. After the micellar solution is passed through a 0.22 μm microporous membrane, the preservative benzalkonium chloride and the isotonic regulator sodium chloride are added, the pH is adjusted to 7.4, and the sterile filter is then dispensed. The average particle diameter of the dihydromyricetin micelles measured by the laser particle size analyzer is 10.1 nm, the dispersion index PDI=0.015, and the dispersion uniformity is good. The dihydromyricetin micelle eye drop is stored at room temperature for 3 months, and the leakage of dihydromyricetin from the micelle is less than 10%. ...
Embodiment 2
[0026] Put 20mg of dihydromyricetin and 300mg of rebaudioside A in a 100mL round-bottomed flask, add 50mL of absolute ethanol, after fully dissolving, evaporate the absolute ethanol in a 40°C water bath in a rotating vacuum to obtain a uniformly dispersed film of the drug and the copolymer , then add distilled water, fully wash the membrane, 300w power water bath ultrasonic 10min, get micellar solution. After the micellar solution is passed through a 0.22 μm microporous membrane, the preservative benzalkonium chloride and the isotonic regulator sodium chloride are added, the pH is adjusted to 7.4, and the sterile filter is then dispensed. The average particle size of the dihydromyricetin micelles measured by the laser particle size analyzer is 10.7nm, the dispersion index PDI=0.021, and the dispersion uniformity is good. The dihydromyricetin micelle eye drop is stored at room temperature for 2 months, and the leakage of dihydromyricetin from the micelle is less than 10%.
Embodiment 3
[0028] Put 100mg of dihydromyricetin and 3000mg of rebaudioside A in a 100mL round-bottomed flask, add 50mL of absolute ethanol, after fully dissolving, evaporate the absolute ethanol in a 40°C water bath in a rotating vacuum to obtain a uniformly dispersed film of the drug and the copolymer , then add distilled water, fully wash the membrane, 300w power water bath ultrasonic 10min, get micellar solution. After the micellar solution is passed through a 0.22 μm microporous membrane, the preservative benzalkonium chloride and the isotonic regulator sodium chloride are added, the pH is adjusted to 7.4, and the sterile filter is then dispensed. The average particle diameter of the dihydromyricetin micelles measured by the laser particle size analyzer is 10.5nm, the dispersion index PDI=0.019, and the dispersion uniformity is good. The dihydromyricetin micelle eye drop is stored at room temperature for 2.5 months, and the leakage of dihydromyricetin from the micelle is less than 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com