125I-labeled Caerin polypeptide and application thereof
A technology of labeling and polypeptide solutions, applied in the application of medicines, in the field of preparation and treatment of tumors, to achieve the effects of increasing in vitro storage time, good in vitro stability, reducing preparation costs and chemical toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1125I
[0042] Example 1 125 Preparation of I-labeled F3 polypeptide
[0043] S1. Take lodogen, add chloroform to dissolve it at a solid-to-liquid ratio of 1 mg / mL, take 100 μL and add it to the bottom of the reaction tube, dry it under negative pressure, seal it, and store it at -15°C to -20°C to prepare a reaction coated with lodogen. Tube;
[0044] S2. Take the reaction tube coated with lodogen prepared in step S1, and add 40 μL of F3 polypeptide solution with a concentration of 0.4 μg / μL and 20 μL of Na with a concentration of 25 μCi / μL in sequence. 125 1, stirring reaction 15min under the condition of 25 ℃ at temperature;
[0045] S3. After the reaction time is over, immediately add 300 μL of phosphate buffer solution with a concentration of 0.05 mol / L and a pH of 7.4 to terminate the reaction, and let stand for 5 minutes to obtain a reaction solution;
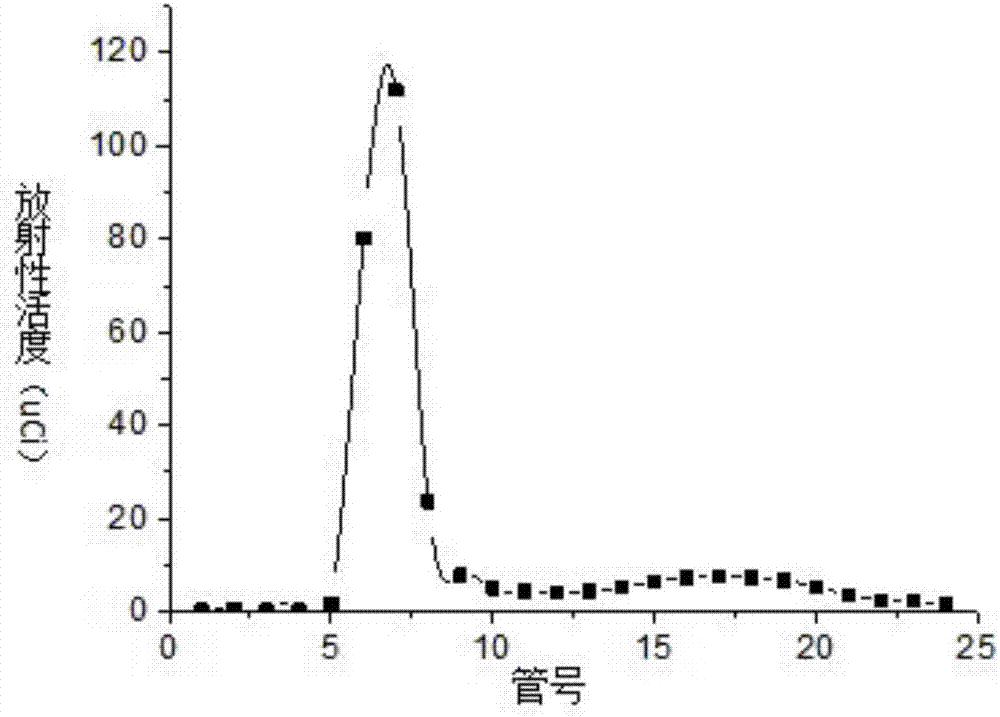
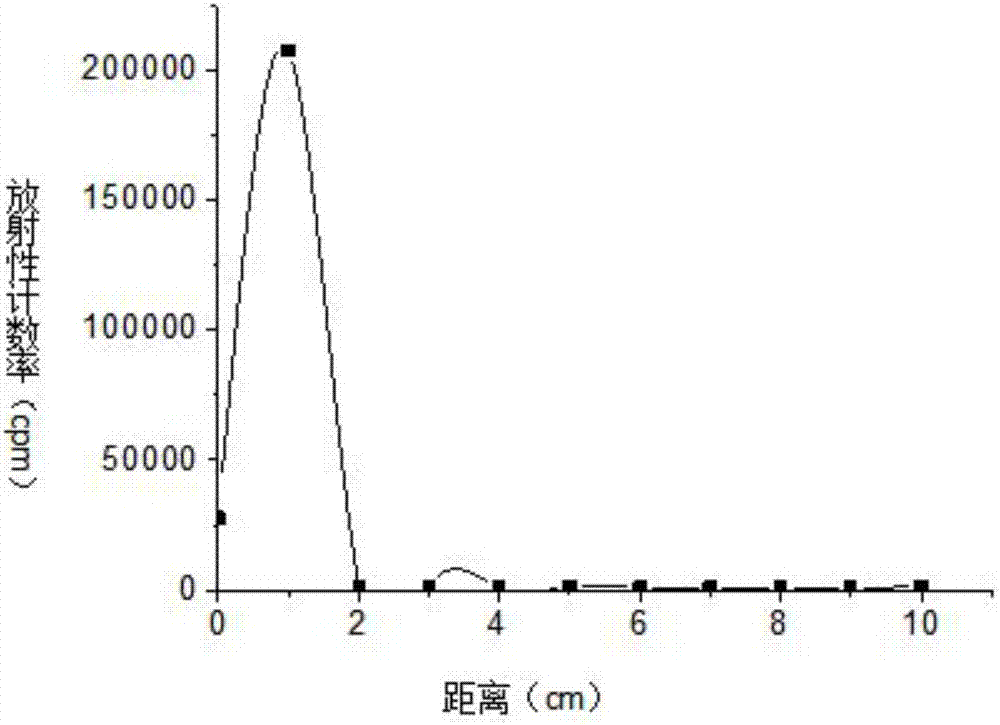
[0046] S4. Purify the reaction solution described in step S3 with a SephadexG-25 chromatography column, elute with a phospha...
Embodiment 2125I-F3
[0050] Example 2 125 I-F3 Stability Investigation
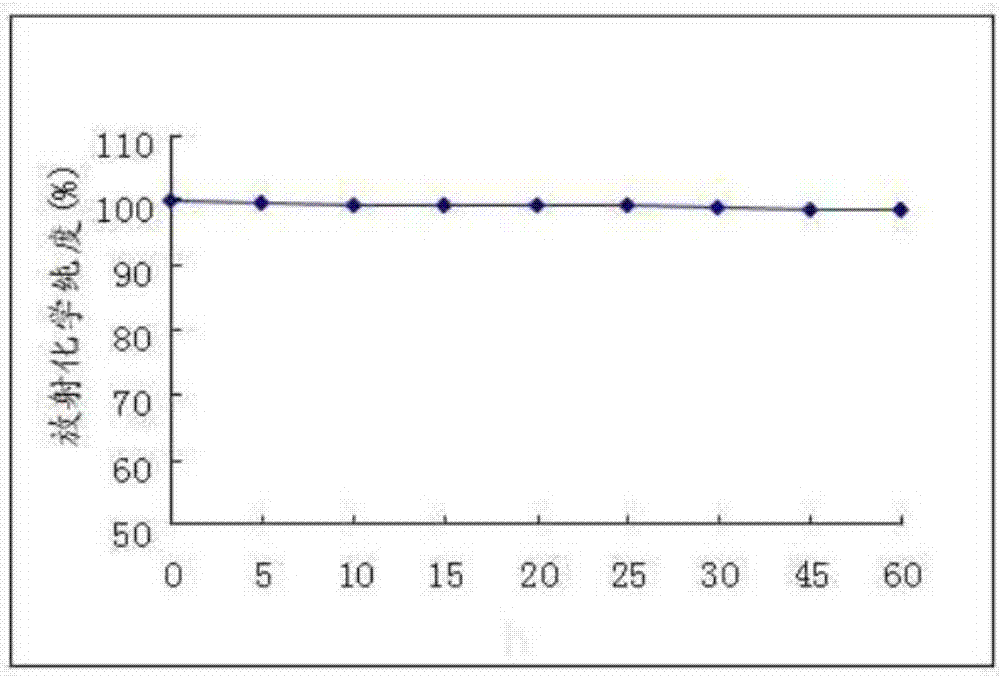
[0051] (1) take 125 I-F3, placed under the condition of 4°C for 60h, measured respectively after 0h, 5h, 10h, 15h, 20h, 25h, 30h, 45h, 60h, 125 The radiochemical purity of I-F3, see image 3 . Depend on image 3 The results show that the present invention provides 125 After I-F3 was stored at 4°C for 60 hours, the radiochemical purity was still as high as 98.25%.
[0052] (2) take 125 I-F3, placed under the condition of 25 ℃ for 24h, measured respectively after 2h, 4h, 8h, 12h, 16h, 20h, 24h, 125 The radiochemical purity of I-F3, see Figure 4 . Depend on Figure 4 The results show that the present invention provides 125 After I-F3 was stored at 25°C for 24 hours, the radiochemical purity was still as high as 98.01%.
[0053] (3) Take 50 μL of 125 I-F3 was added to 100 μL of normal saline and 100 μL of human plasma, and placed at a temperature of 37°C for 48 hours. After 10 minutes, 20 minutes, 30 minutes, 1 hour...
Embodiment 3125I-F3
[0054] Example 3 125 Inhibitory effect of I-F3 on the growth of MCF-7 cells in vitro
[0055] Take the MCF-7 cells (human breast cancer cells) in the logarithmic growth phase, adjust the cell concentration to 1×10 with the RPMI1640 medium containing 10% volume fraction calf serum 5 / mL, seeded in a 96-well plate, 100 μL per well, and 6 parallel wells for each group. After inoculation, different concentrations of 125 I-F3 medium 10 μL / well, 125 The radioactivity of I-F3 was 1, 10, 37, 100, 200 and 500kBq / well, and the control group Na 125 The I radioactivity is 1, 10, 37, 100, 200, 500kBq / well, and the blank group is an equal-volume culture medium without cells, placed at 37°C and 5% CO 2 After culturing in the incubator for 24 and 48 hours, add 10 μL / well of MTT (5 mg / mL), continue to incubate for 4 hours, then terminate the culture, add 10% SDS-HCl 100 μL / well, and act at 37°C for 12 hours. Select a wavelength of 570nm, measure the optical absorption value (OD) of each w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com