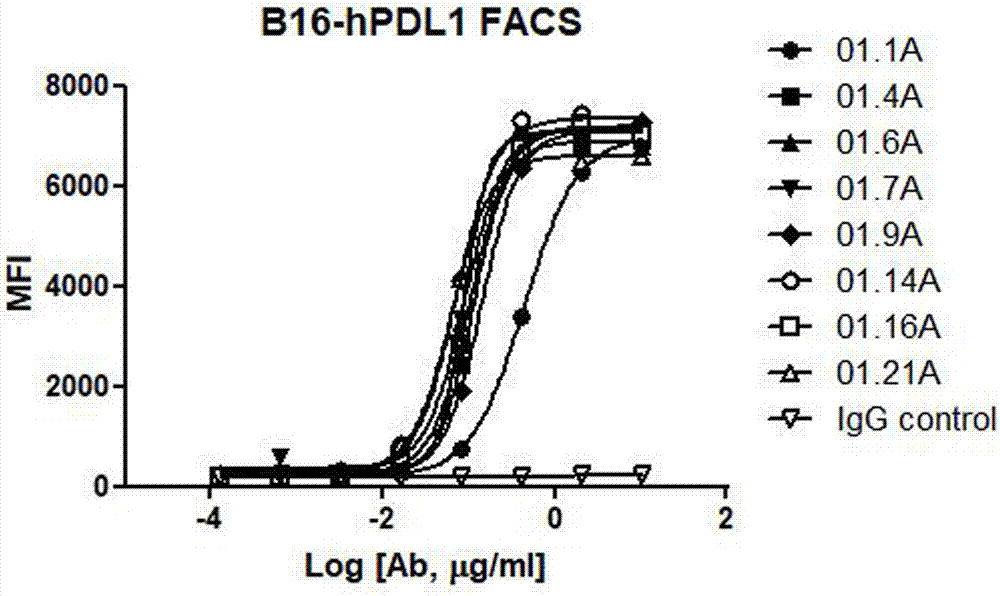
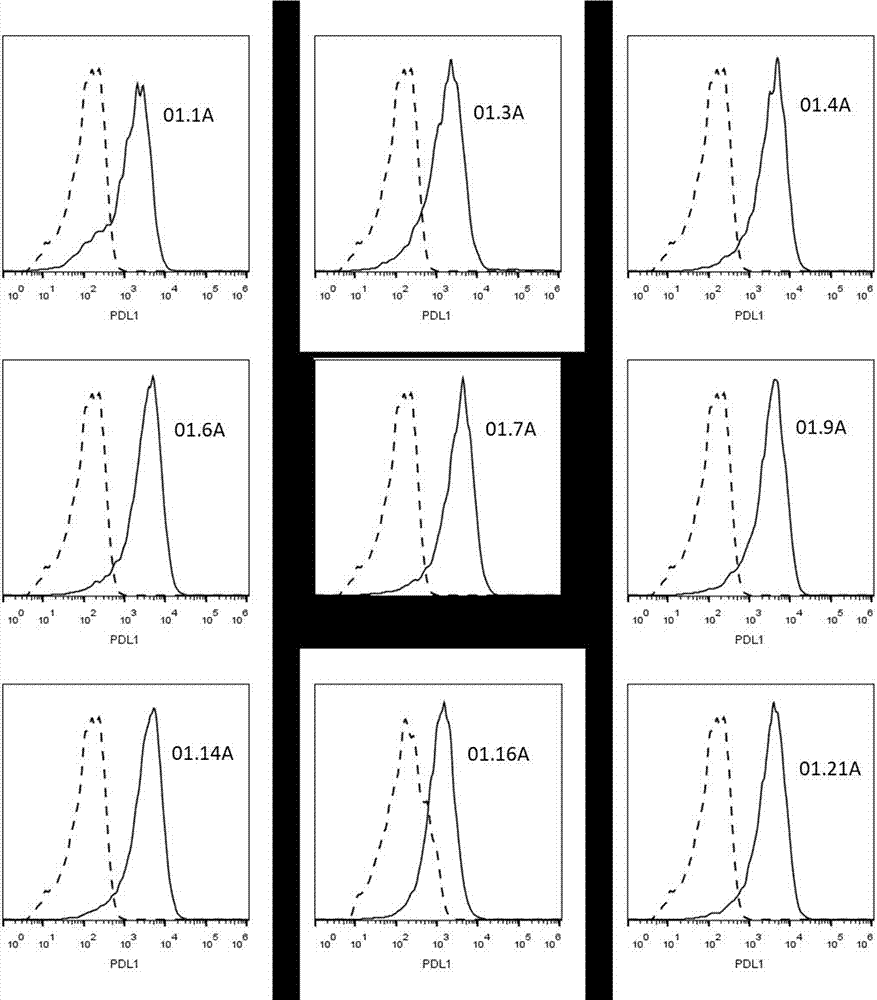
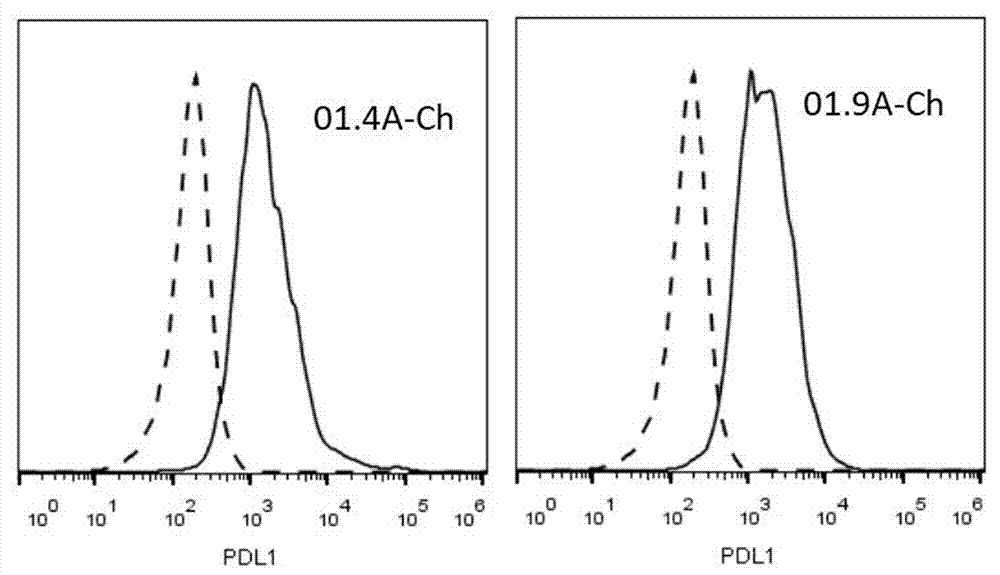
PDL1 monoclonal antibody and application thereof
An antibody and antibody heavy chain technology, applied in the field of tumor treatment and molecular immunology, to achieve the effect of strengthening in vitro biological activity, promoting immune function and reducing immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1: Animal immunization and screening of anti-PDL1 mouse antibody
[0109] Balb / c mice of appropriate age were selected for immunization. After hPDL1-mFc fusion protein was used as antigen mixed with complete Freund's adjuvant (Sigma-Aldrich), it was injected subcutaneously into immunized mice to stimulate corresponding B lymphocyte clones. Immunized mice were then boosted by intraperitoneal injection of 100 μg of hPDL1-mFc emulsified 1:1 in incomplete Freund's adjuvant (Sigma-Aldrich) approximately every two to three weeks. Remove mouse spleen lymphocytes through aseptic operation, and prepare SP2 / 0 myeloma cells in a certain ratio (spleen cells 1x10 8 , myeloma cells 2x10 7 ) were mixed, and polyethylene glycol (Sigma, P7181) was added for cell fusion.
[0110] After fusion, add fused cells to a 96-well plate, add 0.1 mL HAT medium to each well, put them in a carbon dioxide incubator, and culture at 37°C; on the 4th day, add 0.1 mL HT medium to each well; ...
Embodiment 2
[0116] Example 2: Cloning of murine antibody cDNA and construction of chimeric antibody
[0117] The variable region gene sequences of the heavy chain and light chain of the hybridoma antibody were obtained by using degenerate primer PCR method. Hybridoma monoclonal cells were lysed with Trizol (Invitrogen, catalog #15596-018) to isolate total RNA, and the SuperScript III First-Strand Synthesis System (Invitrogen, catalog #18080-051) was used for reverse transcription using RNA as a template , to obtain a cDNA library. Using the obtained cDNA library as a template, PCR was performed using degenerate primers (Zhou H, et al., Nucleic Acids Research 22: 888-889 (1994), Chardes Tet al., FEBS Letters 452: 386-394 (1999)) . The PCR products were detected by agarose gel electrophoresis, and the PCR amplification products of the variable regions of the heavy and light chains were expected to be 400 base pairs in size. The PCR product was cloned into the pClone007 vector (Tsingke,...
Embodiment 3
[0119] Example 3: Kinetic detection of human-mouse PDL1 chimeric antibody
[0120] Using the biomolecular interaction system Octet-96 (Pall Life Sciences, S-000959), the kinetic constant (k assoc and k dissoc ), and further calculate the equilibrium binding constant K D. The hPDL1-mFc antigen protein was coupled to the surface of the AMC sensor (Pall Life Sciences, PN18-5099), and different concentrations of antibodies were added to measure the binding and dissociation between the PDL1 chimeric antibody and the PDL1-mFc protein on the sensor surface. Specifically, the AMC sensor was pre-wetted in the buffer (PBS containing 0.02% Tween-20 and 0.1% BSA) for 10 min, and then equilibrated in the sample buffer of hPDL1-mFc for 5 min, so that the PDL1-mFc protein was coupled to sensor surface. The PDL1-mFc-coupled AMC sensor was first equilibrated in buffer for 2 minutes, then co-incubated in buffer containing different concentrations of antibodies (3-200 nM) for 5 minutes to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com