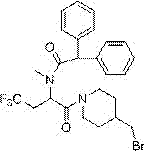
4-benzylpiperidine amide compound and application thereof in controlling plant nematode disease
A benzylpiperidine, plant nematode technology, applied in the application, nematicide, plant growth regulator and other directions, can solve the problems of ecological environment damage, high toxicity, long lasting effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
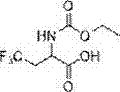
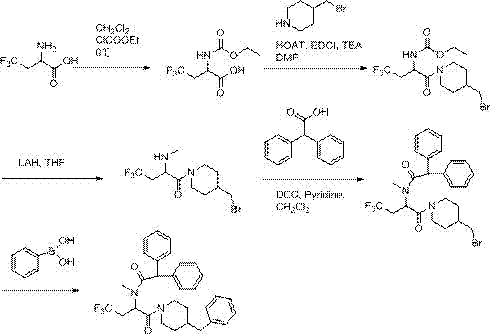
[0021] Example 1: Synthesis of 2-((ethoxycarbonyl)amino)-4,4,4-trifluorobutyric acid
[0022]
[0023] 2-Amino-4,4,4-trifluorobutanoic acid (1.2 g, 7.64 mmol) was dissolved in anhydrous CH 2 Cl 2 (15 mL) was cooled in an ice bath. Ethyl chloroformate (0.80 mL, 8.40 mmol) was added and the reaction was stirred at 0 °C for 10 minutes. After this time, the reaction was allowed to warm to room temperature and stirring was continued for 2 hours. Evaporate solvent, add 50ml mass fraction in the reactant and be 5% NaHCO 3 The solution was stirred for five minutes, and then 50ml of CH was added to the system 2 Cl 2 Stir for five minutes, separate the layers, and wash the aqueous layer with 50 mL of CH 2 Cl 2 were extracted twice, and then the combined organic layers were washed with anhydrous MgSO 4 The crystals were dried and concentrated to obtain 2-((ethoxycarbonyl)amino)-4,4,4-trifluorobutanoic acid as a white solid, yield 1.69 g, 7.40 mmol, yield 96.8%. 1 H-NMR (400 M...
Embodiment 2
[0024] Example 2: Synthesis of (1-(4-(bromomethyl)piperidin-1-yl)-4,4,4-trifluoro-1-oxobutane-2-yl)carbamate tert-butyl ester
[0025]
[0026] 2-((ethoxycarbonyl)amino)-4,4,4-trifluorobutanoic acid (1.3g, 5.67mmol) obtained in Example 1, 4-(bromomethyl)piperidine (0.75g, 4.21 mmole), HOAT (0.74 g, 5.47 mmole), EDCI (1.05 g, 5.47 mmole) and triethylamine (1.4 mL, 10 mmole) were dissolved in DMF (30 mL) and stirred at room temperature overnight. All volatiles were distilled off under high vacuum, and the residue was dissolved in a 4:1 chloroform / isopropanol mixture solvent. The organic solution was washed twice with brine and washed with anhydrous MgSO 4 Dry and concentrate in vacuo. flash chromatography on silica gel (SiO 2 , DCM:MeOH:10% ammonia solution=80:10:10) to give white solid (1-(4-(bromomethyl)piperidin-1-yl)-4,4,4-trifluoro-1 -Oxobutan-2-yl) tert-butyl carbamate, 1.29 g, 92% yield. 1 H-NMR (400 MHz, CDCl3) δ: 1.04(t, 3H), 1.51(m, 2H), 1.79-2.08(m, 3H), 2.67(...
Embodiment 3
[0027] Example 3: Synthesis of 1-(4-(bromomethyl)piperidin-1-yl)-4,4,4-trifluoro-2-(methylamino)butan-1-one:
[0028]
[0029] At room temperature, under nitrogen atmosphere, to LiAlH 4 (1.2g, 31.62mmol) in tetrahydrofuran solution was added 1-(4-(bromomethyl)piperidin-1-yl)-4,4,4-trifluoro-1-oxobutan-2-yl) A solution of tert-butyl carbamate (1.1 g, 2.83 mmol) in tetrahydrofuran (50 mL). The resulting solution was heated to reflux overnight in an oil bath. The reaction was then quenched by adding 200 mL of ice water. The solid was filtered off. The filtrate was extracted with 2 x 200 mL ethyl acetate. The combined organic layers were washed with Na 2 SO 4 Dry and concentrate in vacuo. Apply the residue to Al 2 o 3On column and eluted sequentially with EtOAc / petroleum ether (5:1), DCM / MeOH (80:1-20:1) to give 1-(4-(bromomethyl)piperidin-1-yl) as a yellow solid -4,4,4-Trifluoro-2-(methylamino)butan-1-one, 0.89 g, 95% yield. 1 H-NMR (400 MHz, CDCl3) δ:1.39-1.51(m, 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com