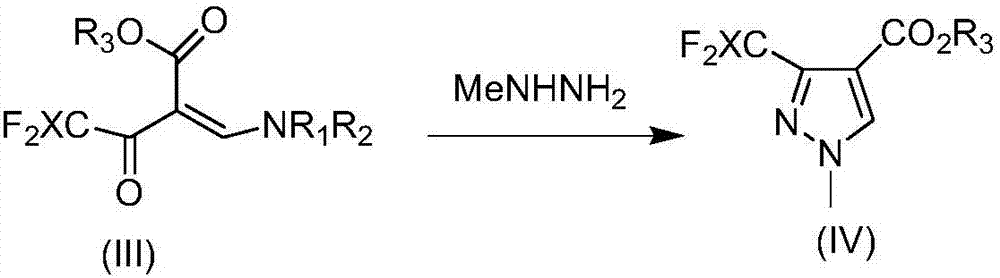Method for preparing 3-difluoromethyl-1-methyl-1H-pyrazol-4-carboxylic acid
A difluoromethyl, C1-C12 technology, applied in the field of preparation of 3-difluoromethyl-1-methyl-1H-pyrazole-4-carboxylic acid, can solve the problem of harsh process conditions, low yield, Industrial scale-up production is difficult and other problems, to achieve the effect of significant technological progress and high productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
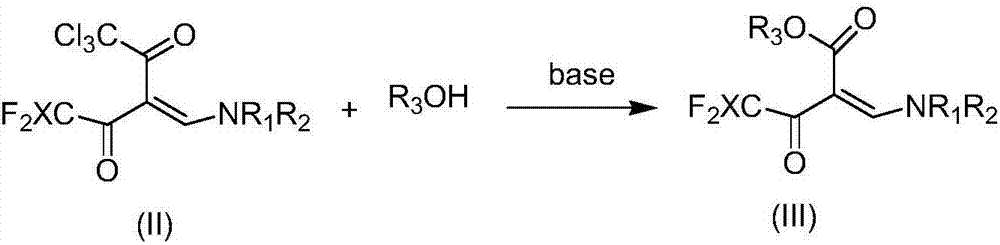
[0039] Preparation of 2-(dimethylaminomethylene)-4,4-difluoro-3-oxo-butyric acid hexyl ester
[0040]
[0041] 1,1,1-trichloro-5,5-difluoro-3-(dimethylaminomethylene)-2,4-pentanedione (10.0mmol, 2.81g) K 2 CO 3 (20.0mmol, 2.76g) and acetonitrile (5ml) mixture was stirred at room temperature for 17h, then cooled to 0°C, concentrated hydrochloric acid was added to adjust pH=1, extracted 3 times with ethyl acetate (10ml), the organic phases were combined, After drying with anhydrous magnesium sulfate and concentrating in vacuo, the target product was obtained quantitatively (HPLC analysis), and the yield was 100%.
Embodiment 2
[0043] Preparation of 3-(difluoromethyl)-1-methyl-1H-pyrrolyl-4-carboxylic acid hexyl ester
[0044]
[0045] An aqueous solution of methylhydrazine (40%, 12mmol, 1.38g), 48% NaOH (2mmol, 167mg) and dichloromethane (5ml) were mixed and cooled to -15°C, and added to 2-(dimethyl Aminomethylene)-4,4-difluoro-3-oxo-butyric acid hexyl ester (10mmol, 2.77g) in dichloromethane solution (5ml). The reaction was continued at the same temperature for 4 h, then neutralized to neutral by adding 2N HCl, the temperature was raised to room temperature, extracted three times with dichloromethane, the organic phase was combined, washed with water, dried with anhydrous magnesium sulfate, concentrated under reduced pressure . The target product was purified by column chromatography with a yield of 85%.
Embodiment 3
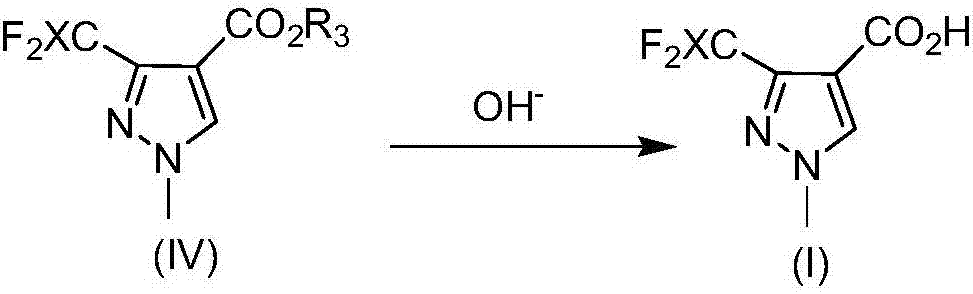
[0047] Preparation of 3-Difluoromethyl-1-methyl-1H-pyrazole-4-carboxylic acid
[0048]
[0049] 3-(Difluoromethyl)-1-methyl-1H-pyrrolyl-4-carboxylic acid hexyl ester (5mmol, 1.30g) and 10% NaOH aqueous solution (8mmol, 3.10g) were stirred at 60°C for 2h. The reaction Cool to room temperature, and adjust pH to 1 by adding concentrated hydrochloric acid. The precipitate was filtered, washed with cyclohexane, and dried in vacuo to obtain pure 3-difluoromethyl-1-methyl-1H-pyrazole-4-carboxylic acid in 95% yield.
[0050] The above examples show that, according to the preparation method provided by the present invention, 3-difluoromethyl-1-methyl-1H-pyrazole-4-carboxylic acid can be generated with high selectivity. The whole implementation process is simple and easy to operate, can meet technical and economic requirements, and is friendly to the environment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com