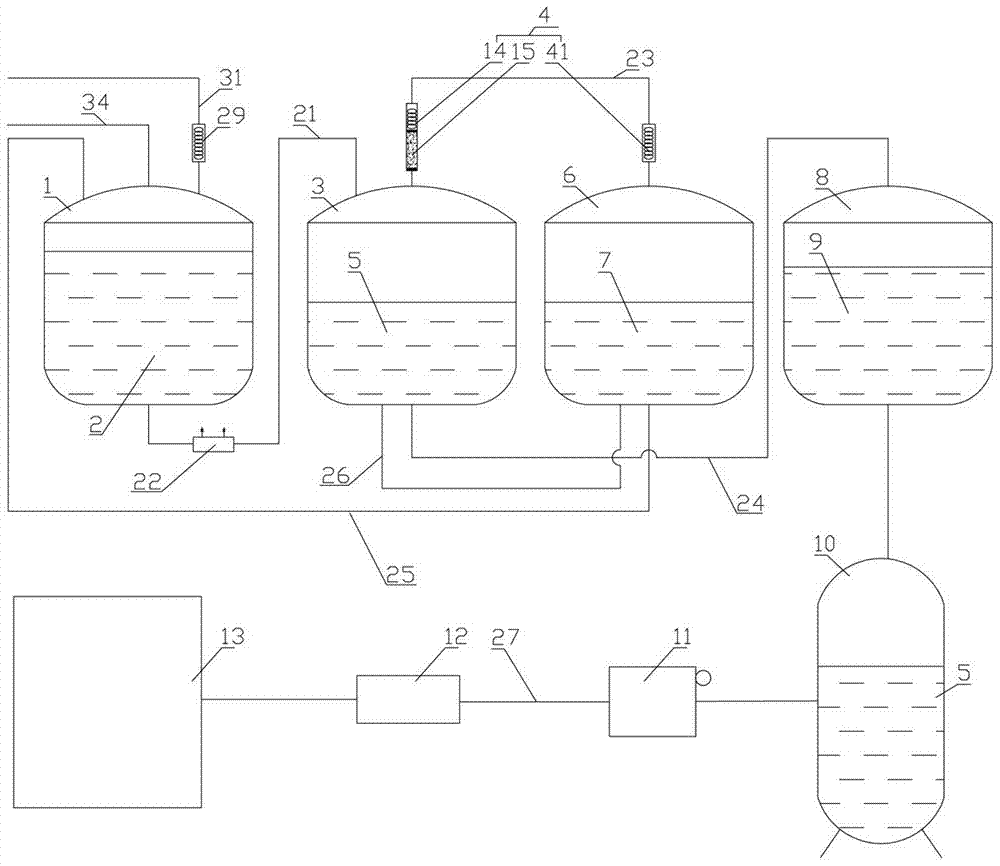
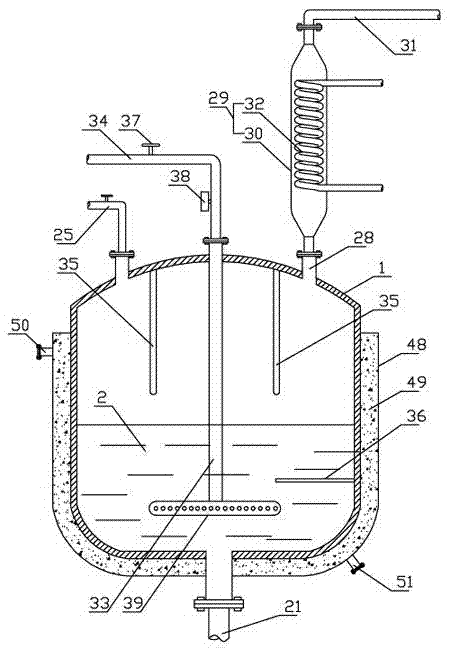
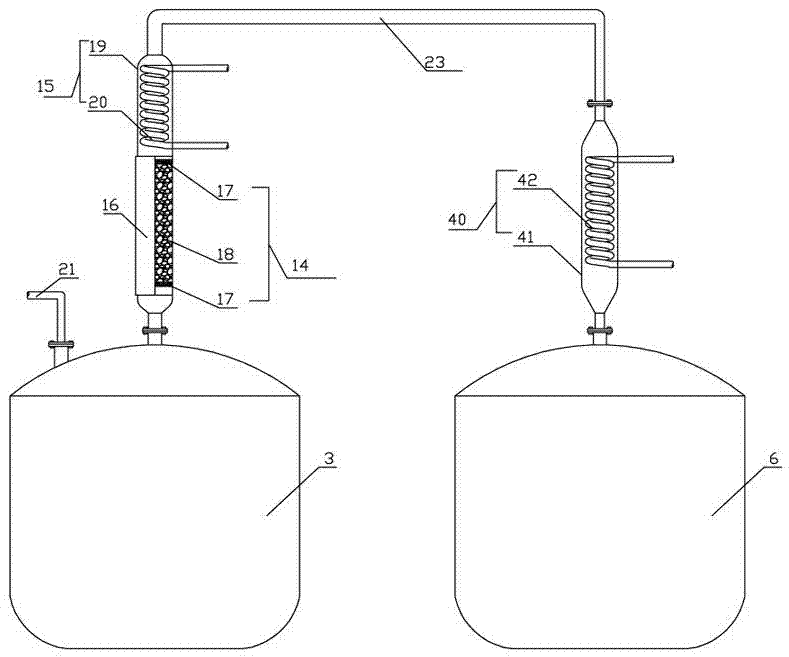
Synthesizing method and device for o-nitrochlorobenzene
The technology of a nitrile benzyl chloride and a synthesis method, which is applied in the field of organic synthesis methods and devices thereof, can solve the problems of low cost, high cost, heavy pollution product purity, etc., and achieves reduction of suction filtration process, improvement of safety, and shortening of fractionation time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] In the present embodiment, a kind of synthetic method of o-cyano benzyl chloride comprises the following steps:
[0052] In the first step, the o-methylbenzonitrile liquid is passed into the reactor 1 at a speed of 30 kg per hour, and the temperature in the reactor 1 is slowly raised to 125° C., the ultraviolet light source 35 in the reactor 1 is opened, and The speed of 10kg per hour is passed into chlorine in the reactor 1;
[0053] In the second step, when the feed rate of the o-methylbenzonitrile liquid in the reactor 1 reached 45% of the internal volume of the still, the feed of the o-tolunitrile liquid and chlorine gas was stopped, and after leaving standstill for 0.5 hour, then According to the feed speed of o-methyl benzonitrile liquid and chlorine in the first step, in reactor 1, pass into the o-methyl benzonitrile liquid and the chlorine of the first step, the o-methyl benzonitrile liquid and the first step After the introduction of chlorine gas is completed,...
Embodiment 2
[0072] In the present embodiment, in the present embodiment, a kind of synthetic method of o-cyano benzyl chloride comprises the following steps:
[0073] In the first step, the o-methylbenzonitrile liquid is passed into the reactor 1 at a speed of 30 kg per hour, and the temperature in the reactor 1 is slowly raised to 130° C., the ultraviolet light source 34 in the reactor 1 is opened, and The speed of 11kg per hour is passed into chlorine in reactor 1;
[0074] In the second step, when the feed rate of the o-methylbenzonitrile liquid in the reactor 1 reached 45% of the internal volume of the still, the feed of the o-tolunitrile liquid and chlorine gas was stopped, and after leaving standstill for 0.5 hour, then According to the feed speed of o-methyl benzonitrile liquid and chlorine in the first step, in reactor 1, pass into the o-methyl benzonitrile liquid and the chlorine of the first step, the o-methyl benzonitrile liquid and the first step After the introduction of chl...
Embodiment 3
[0092] In the present embodiment, in the present embodiment, a kind of synthetic method of o-cyano benzyl chloride comprises the following steps:
[0093] In the first step, the o-methylbenzonitrile liquid is passed into the reactor 1 at a speed of 30 kg per hour, and the temperature in the reactor 1 is slowly raised to 135° C., the ultraviolet light source in the reactor 1 is turned on, and every The speed of hour 12kg is passed into chlorine in reactor 1;
[0094] In the second step, when the feed rate of the o-methylbenzonitrile liquid in the reactor 1 reached 45% of the internal volume of the still, the feed of the o-tolunitrile liquid and chlorine gas was stopped, and after leaving standstill for 0.5 hour, then According to the feed speed of o-methyl benzonitrile liquid and chlorine in the first step, in reactor 1, pass into the o-methyl benzonitrile liquid and the chlorine of the first step, the o-methyl benzonitrile liquid and the first step After the introduction of c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com