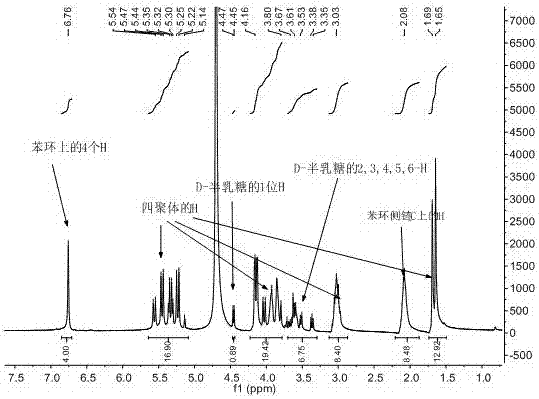
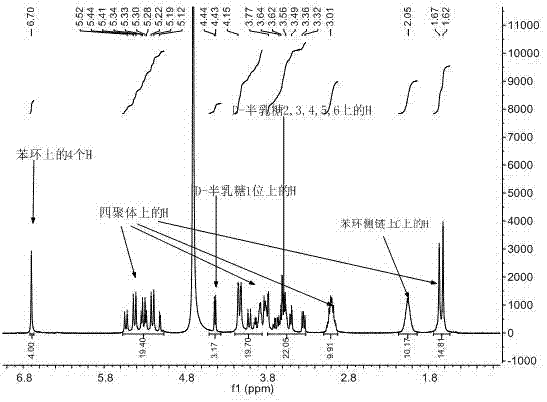
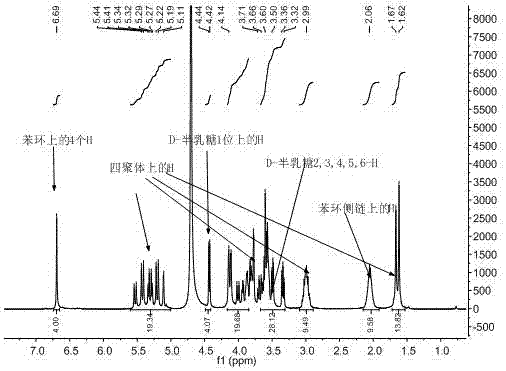
D-galactose-bonded open-ringed cucurbituril and application thereof
A technology of cucurbituril and galactose, which is applied in the field of supramolecular drug carriers, can solve the problems of not being able to achieve targeted transportation, not having molecular targeting, and not being able to identify and assemble, and achieve the effects of improving solubility and water solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the synthetic preparation of tetramer
[0028] With reference to the method in the literature Ma D, et al. Nature Chemistry, 2012, 4(6):503:
[0029] (1) Synthesis of dimer: Mix glycoluril (500 g, 3.51 mol) and paraformaldehyde (105 g, 3.51 mol) evenly and add it to HCl solution (8 M, 700 mL). Heated for 48 hours; after the reaction was completed, the reaction solution was cooled and vacuum filtered to obtain a crude product; the crude product was washed with water (500 mL), and then recrystallized with TFA (1.5 L) to obtain a dimer as a white solid ( 334g, 62%);
[0030] ;
[0031] (2) Synthesis of tetramer: Dimer (304 g, 1.20 mol) was added to anhydrous MeSO 3 H (600 mL), after the dimer was completely dissolved and the solution became transparent, then etherified methyl glycoluril (84 g, 0.27 mol) was added; the reaction solution was reacted at 50°C for 3 hours; the reaction was completed Finally, the reaction solution was poured into water (6.0 L...
Embodiment 2
[0033] Embodiment 2: the synthesis of bromobenzene ring side arm
[0034] Referring to the method in the literature Zhang M, Sigwalt D, Isaacs L. [J]. Chemical Communications, 2015,51(78):14620-3, add hydroquinone bis(2-hydroxyethyl) ether in acetonitrile (5 mL) (1.98 g, 0.01 mol), add carbon tetrabromide (13.24 g, 0.04 mol) and triphenylphosphine (5.24 g, 0.02 mol) after hydroquinone bis(2-hydroxyethyl) ether is completely dissolved, and the reaction solution React at room temperature for 4 h. After the reaction, pure water (200 mL) was added to the reaction solution to produce a white precipitate, which was vacuum filtered to obtain a crude product; the crude product was washed 3 times with methanol / water (volume ratio 3:2), each 100 mL each time, the pure product was obtained. Dry in a vacuum drying oven to obtain a white solid, and finally obtain the side arm of the brominated benzene ring;
[0035] .
Embodiment 3
[0036] Embodiment 3: the synthesis of brominated open-ring cucurbituril
[0037] Weigh 1.8 g (2.45 mmol) of the tetramer, add 8 mL of acetic anhydride and 8 mL of trifluoroacetic acid to the tetramer, and mix thoroughly in an oil bath at 70°C until the tetramer is completely dissolved, then Add 1.6 g (4.9 mmol) of the side arm of the bromobenzene ring and continue the reaction for 3 h;
[0038] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com