Compound, preparation method and application thereof in preparation of medicine for preventing/curing autoimmune diseases
An autoimmune and compound technology, applied in the field of medicine, can solve problems affecting the quality of life of patients, joint destruction, deformity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
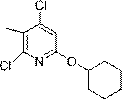
[0032] Example 1: Synthesis of 2,4-dichloro-6-(cyclohexyloxy)-3-methylpyridine
[0033]
[0034] To a solution of sodium hydride NaH (4.51 g, 0.188 mmol, 60 wt % in mineral oil) in THF (200 mL) was added cyclohexanol (15.6 mL, 0.15 mol) dropwise at 0 °C. After stirring at 0°C for 30 minutes, a THF (40 mL) solution of 2,4,6-trichloro-3-methylpyridine (Compound 1) (26.52 g, 0.135 mol) was added dropwise via syringe. The reaction mixture was warmed to room temperature and stirred for 4 hours. The reaction was cooled to 0°C, and saturated aqueous ammonium chloride solution was slowly added to quench the reaction. The reaction mixture was allowed to warm to room temperature, diluted with ethyl acetate, and washed with saturated aqueous sodium bicarbonate and saturated aqueous sodium thiosulfate. The organic layer was separated and the aqueous layer was extracted twice with ethyl acetate. The combined organic layers were dried over sodium sulfate, filtered and concentrated. P...
Embodiment 2
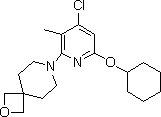
[0035] Example 2: Synthesis of 7-(4-chloro-6-(cyclohexyloxy)-3-methylpyridin-2-yl)-2-oxa-7-azaspiro[3.5]nonane
[0036]
[0037] A 2L three-necked flask equipped with a mechanical stirrer, a thermometer and a dropping funnel was charged with ethanol (375mL), water (375mL) and 2-oxa-7-azaspiro[3.5]nonane (19.08g, 0.15 mol), the resulting solution was cooled (with an ice-salt bath) to about 0°C, and a solution of compound 2 (33.37g, 0.128mol) in ethyl acetate (47.5mL) was added dropwise within about 20 minutes, keeping the temperature low at 10°C. The dropping funnel was rinsed twice with ethyl acetate (20 mL), and the rinses were transferred to the reaction mixture. The reaction was checked by TLC to determine when the reaction was complete. After the reaction, ice water (375 mL) was added and stirred for 30 minutes to complete the precipitation. The white solid was filtered off, washed 6 times with water (225 mL for each wash), and dried under vacuum at 40-50 °C until a ...
Embodiment 3
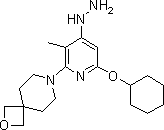
[0038] Example 3: Synthesis of 7-(6-(cyclohexyloxy)-4-hydrazino-3-methylpyridin-2-yl)-2-oxa-7-azaspiro[3.5]nonane
[0039]
[0040] Under nitrogen purging, the suspension of compound 3 (41.95g, 0.120mol) synthesized in Example 2 and hydrazine monohydrate (7.51g, 0.15mol) in dioxane (255mL) was boiled and refluxed for 6 Hour. Ice water (400 mL) was added to the reaction mixture and left overnight. The resulting precipitate was filtered off, washed 3 times with water (260 mL each), and dried under vacuum at 40-50 °C until constant weight to give 7-(6-(cyclohexyloxy)-4-hydrazino-3-methanol ylpyridin-2-yl)-2-oxa-7-azaspiro[3.5]nonane (compound 4), 27.77g, yield 66.8%. 1 H-NMR (400 MHz, CDCl 3 ) δ: 1.08-1.29(m, 5H),1.58-1.69(m, 3H), 1.84(t, 4H), 1.88-1.98(m, 4H),2.31(s, 1H), 2.40(s, 3H) ,3.71(t, 4H), 4.17(m, 1H), 4.72(s, 4H), 5.21(s, 1H). 13 C-NMR (125 MHz, CDCl 3 )δ: 11.50, 24.60, 25.92, 28.55, 30.44, 38.75, 45.85, 76.82, 81.26, 85.95, 109.94, 147.44, 161.81, 164.78. LC-M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com