Synthetic method of houttuynin heterozygous flavonoid compound
A technology of hybrid flavonoids and houttuyniatin, which is applied in the field of synthesis of houttuyniatin hybrid flavonoids, can solve the problems of high cost, cumbersome extraction and separation, etc., and achieve the effect of high yield and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
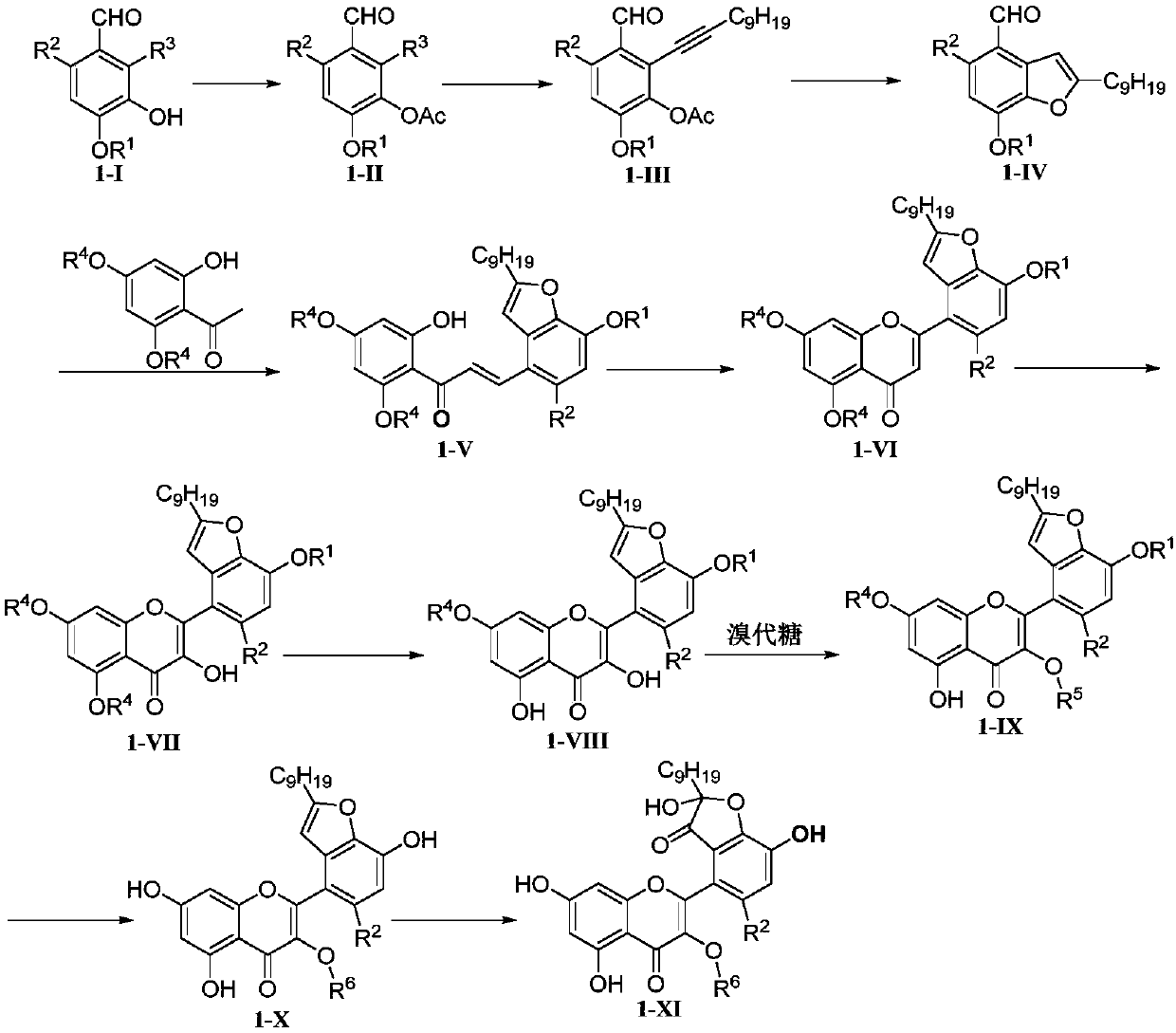
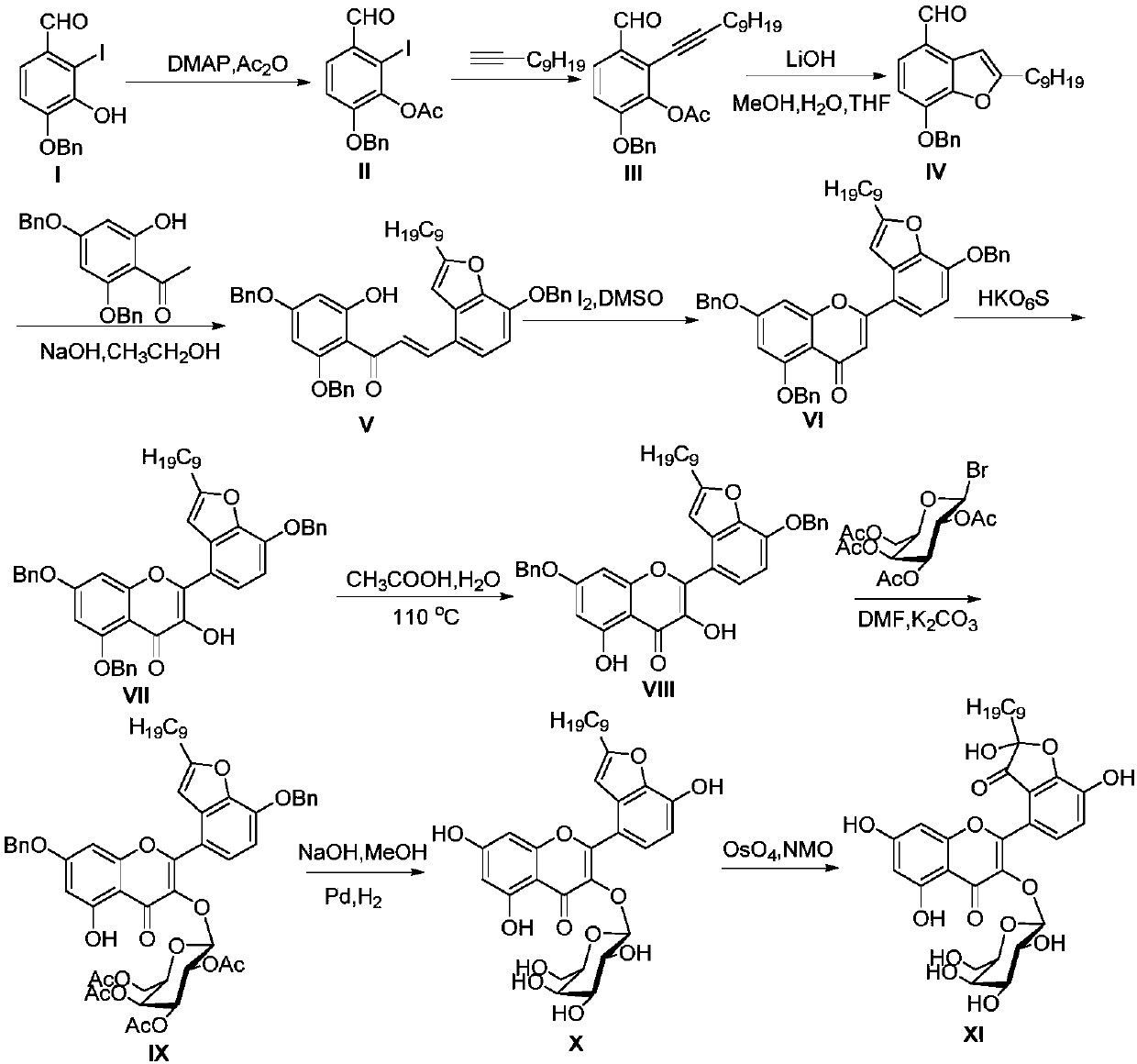
[0104] Example 1: Total synthesis of Houttuynoid G, a hybrid flavonoid of houttuynoid
[0105] route such as figure 2 As shown, the specific steps are as follows:
[0106] ①Preparation of compound II
[0107] References for the preparation method of compound I (Shen, S.D.; Zhang, G.P.; Lei, M.; Hu, L.H. First total synthesis of salvianolic acid C, tournefolic acid A, and tournefolal. ARKIVOC. 2012, 6, 204-213). The obtained 4-benzyl-3-hydroxy-2-iodo-p-hydroxybenzaldehyde was designated as compound I.
[0108] Add 4-benzyl-3-hydroxyl-2-iodo-p-hydroxybenzaldehyde (17g, 48.02mmol), pyridine (Py, 6.8ml), 4-dimethylaminopyridine (DMAP, 293mg, 2.39mmol ), stir. Acetic anhydride (6.8ml) was slowly added dropwise, stirred at room temperature and protected from light for 17h. Remove Py with a rotary evaporator, then adjust the pH to neutral with 10% dilute hydrochloric acid, extract with ethyl acetate (EA), wash the organic phase twice with saturated sodium chloride solution, add...
Embodiment 2
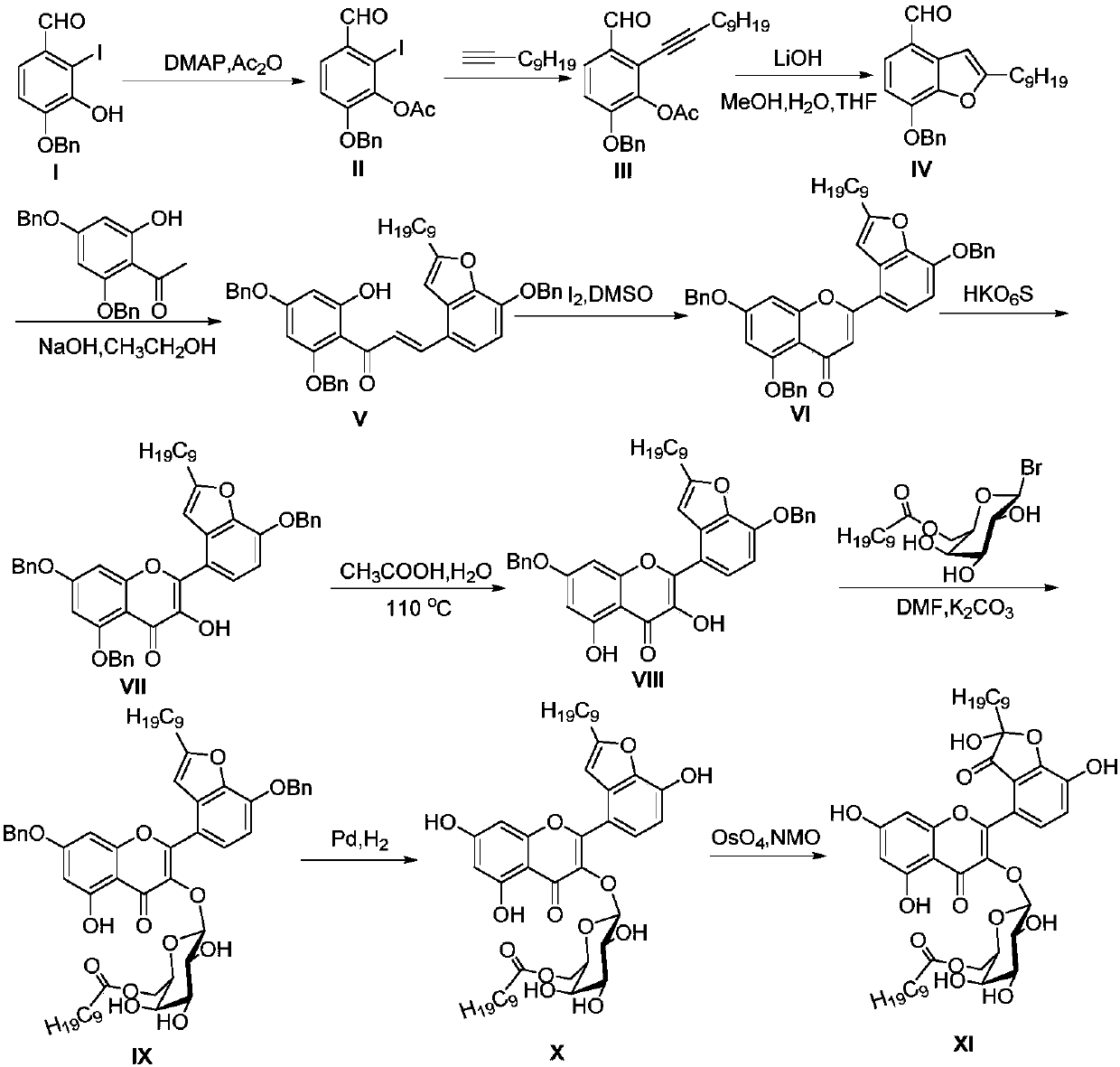
[0137] Example 2: Total Synthesis of Houttuynoid H, a Hybrid Flavone Houttuynoid H
[0138] route such as image 3 As shown, the specific steps are as follows:
[0139] ①Preparation of compound II
[0140] References for the preparation method of compound I (Shen, S.D.; Zhang, G.P.; Lei, M.; Hu, L.H. First total synthesis of salvianolic acid C, tournefolic acid A, and tournefolal. ARKIVOC. 2012, 6, 204-213). The obtained 4-benzyl-3-hydroxy-2-iodo-p-hydroxybenzaldehyde was designated as compound I.
[0141] Add 4-benzyl-3-hydroxyl-2-iodo-p-hydroxybenzaldehyde I (17g, 48.02mmol), pyridine (6.8ml), 4-dimethylaminopyridine (DMAP, 293mg, 2.39mmol) into a 250ml one-port bottle , stir. Acetic anhydride (6.8ml) was slowly added dropwise, stirred at room temperature and protected from light for 17h. Remove Py with a rotary evaporator, then adjust the pH to neutral with 10% dilute hydrochloric acid, extract with ethyl acetate (EA), wash the organic phase twice with saturated sodium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com