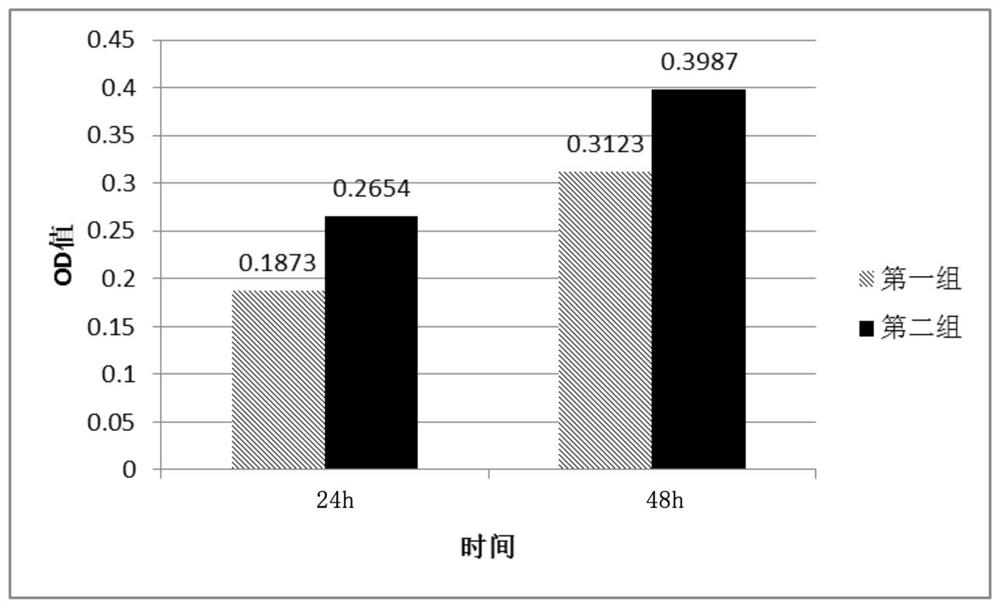
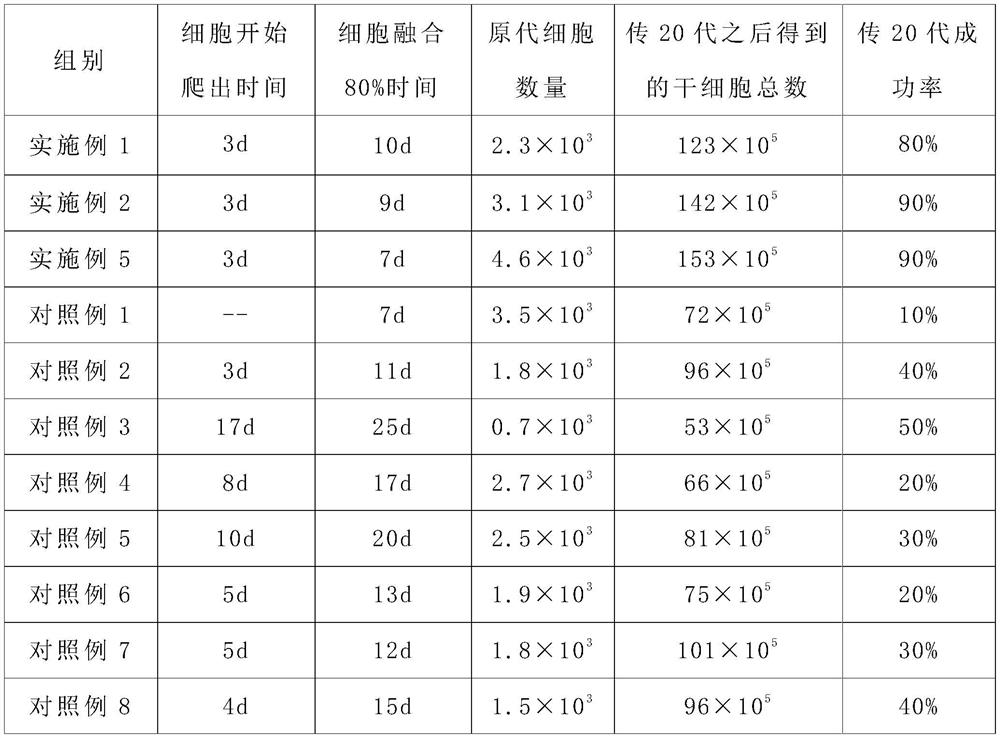
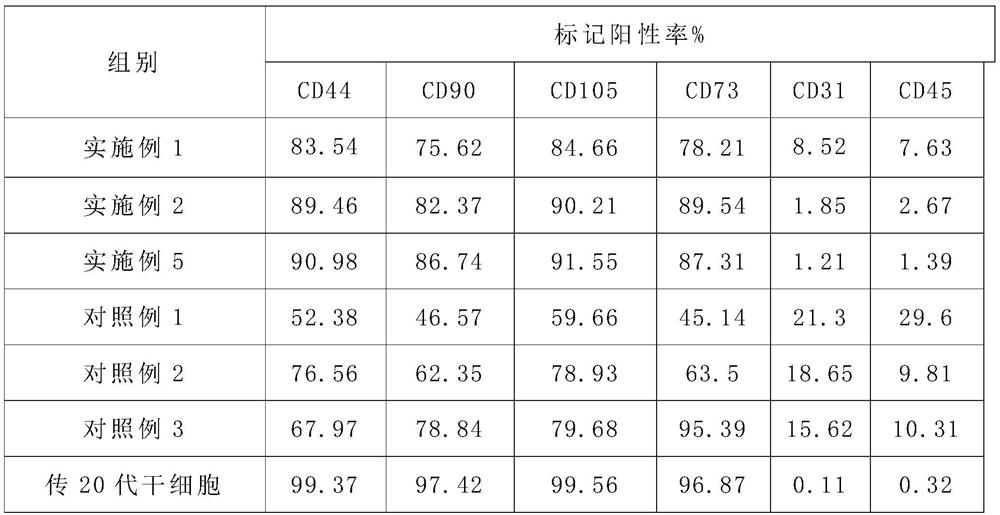
A method for isolating and expanding stem cells after resuscitating umbilical cord tissue and its culture medium
A technology of umbilical cord tissue and stem cells, applied in tissue culture, cell dissociation methods, cell culture active agents, etc., can solve the problems of clinical application limitations of hUCMSCs, poor passaging ability of primary stem cells, and potential safety hazards of heterogeneous serum, etc. Differentiate potential, improve cell resistance to apoptosis, and solve biological safety problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A method for isolating and expanding stem cells after resuscitating umbilical cord tissue, said method comprising the steps of:
[0028] 1) Umbilical cord tissue resuscitation: Take out the cryopreservation tube of frozen umbilical cord tissue, place it in a 37°C water bath to thaw, after thawing, wash it with recovery buffer for 3 times, and obtain the recovered umbilical cord tissue;
[0029] 2) Tissue blocks are obtained; the resuscitated umbilical cord tissue obtained in step 1) is cut into 1mm 3 Tissue blocks were digested by adding digestion solution at 15°C for 1 hour, and then digested at 37°C for 10 minutes. The digestion was terminated, the digestion solution and suspended cells were removed, and undigested tissue blocks were obtained, rinsed in sodium chloride injection, and the supernatant was discarded by centrifugation. The remaining tissue pieces were obtained; wherein, the digestive solution contained 0.5 mg / ml type II collagenase, 0.1 mg / ml TrypLE TM ...
Embodiment 2
[0034] A method for isolating and expanding stem cells after resuscitating umbilical cord tissue, said method comprising the steps of:
[0035] 1) Umbilical cord tissue resuscitation: take out the cryopreservation tube of frozen umbilical cord tissue, place it in a 37°C water bath to thaw, after thawing, wash it with recovery buffer for 5 times, and obtain the recovered umbilical cord tissue;
[0036] 2) Tissue blocks are obtained; the resuscitated umbilical cord tissue obtained in step 1) is cut into 1mm 3 Tissue blocks were digested by adding digestive solution at 15°C for 2 hours, then digested at 37°C for 5 minutes, the digestion was terminated, the digestive solution and suspended cells were removed, undigested tissue blocks were obtained, rinsed in sodium chloride injection solution, and the supernatant was discarded by centrifugation. The remaining tissue pieces were obtained; wherein, the digestive solution contained 0.7mg / ml type II collagenase, 0.2mg / ml TrypLE TM S...
Embodiment 3
[0041] 1) Umbilical cord tissue resuscitation: take out the cryopreservation tube of frozen umbilical cord tissue, place it in a 37°C water bath to thaw, after thawing, wash it with recovery buffer 4 times, and obtain the recovered umbilical cord tissue;
[0042] 2) Tissue blocks are obtained; the resuscitated umbilical cord tissue obtained in step 1) is cut into 1mm 3 Tissue blocks were digested at 15°C for 1.5h by adding digestive fluid, then digested at 37°C for 7min, the digestion was terminated, the digestive fluid and suspended cells were removed to obtain undigested tissue blocks, rinsed in sodium chloride injection, and the supernatant was discarded by centrifugation , to obtain the remaining tissue pieces; wherein, the digestive fluid contains 0.6mg / ml type II collagenase, 0.1mg / ml TrypLE TM Sodium chloride injection of express and 0.4mg / ml prolinephthalein amino acid;
[0043] 3) Isolation of primary stem cells: Place the remaining tissue pieces in a culture dish w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com