Method and device for separating lithium from lithium-containing solution
A lithium solution and lithium ion technology, applied in the field of lithium separation, can solve the problems of high cost, low efficiency, high impurity content, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
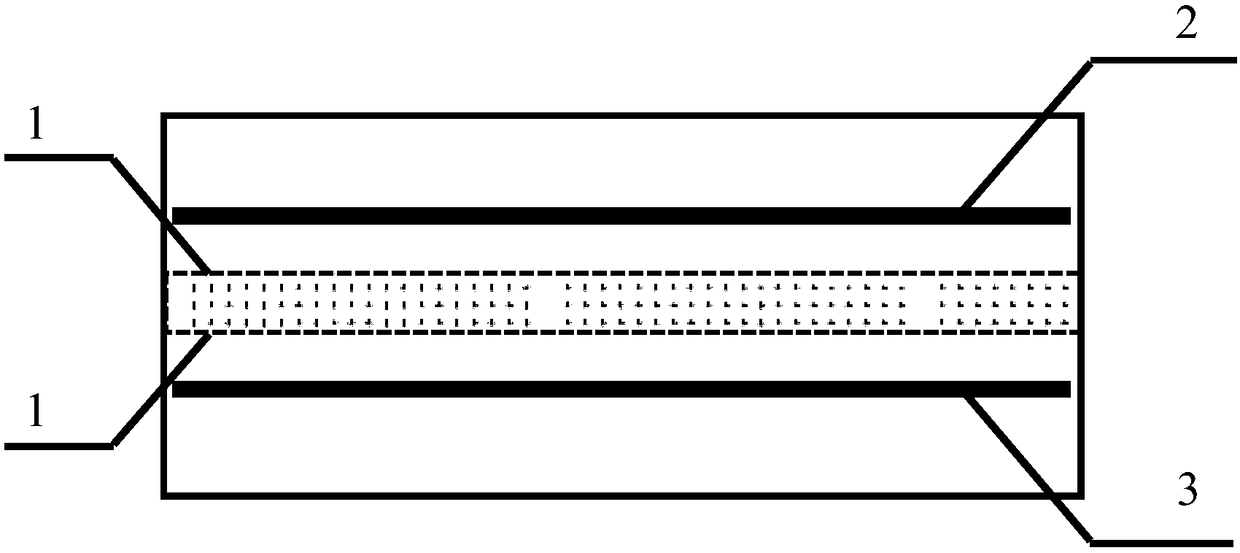
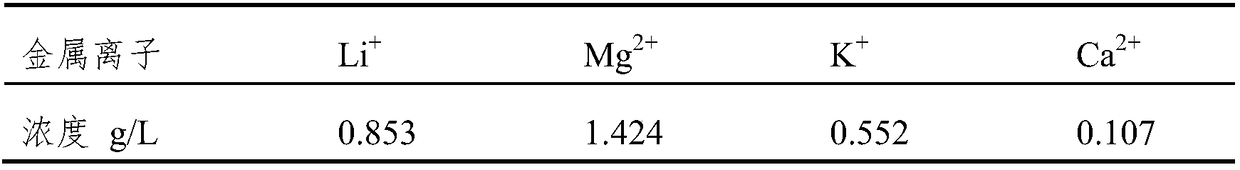
[0055] Using the above-mentioned electrolytic cell, the anode and the cathode are made of carbon fiber cloth, and the two ion exchange membranes are microporous polytetrafluoroethylene membranes. The anode chamber is filled with 300ml of lithium-containing solution, and its cation composition is: Mg 2+ 100g / L, Li + 2g / L, Na + 3.5g / L, K + 3g / L and Ca 2+ 2g / L; the organic phase chamber contains tributyl phosphate and FeCl 3 300ml of supporting electrolyte with a concentration of 1mol / L NaCl is filled in the cathode chamber. The reagents in the three chambers are respectively circulated through the pump, and the current density is controlled at 30°C to 25A / m 2 , keep the pH of the lithium-containing solution in the anode chamber at about 5, and the pH of the solution in the cathode chamber at 2-3. After electrolysis for 10 hours, the concentration of metal ions in the cathode chamber is shown in the table below.
[0056]
Embodiment 2
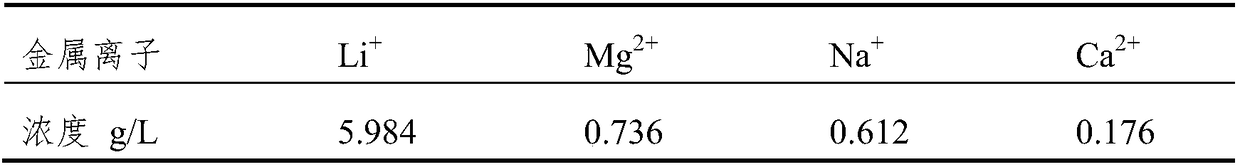
[0058] Using the above-mentioned electrolytic cell, the anode and cathode are made of high-purity graphite, and the two ion-exchange membranes are porous polypropylene membranes. The anode chamber is filled with 300ml of lithium-containing solution, and its cation composition is: Mg 2+ 15g / L, Li + 20g / L, Na + 6g / L, K + 4g / L and Ca 2+ 2g / L, the organic phase chamber is filled with a mixed reagent of tributyl phosphate and dibenzoyltoluene, and the cathode chamber is filled with 300ml concentration of 2mol / L K 2 SO 4 supporting electrolyte. The solutions in the three chambers are respectively circulated through the pump, and the current density is controlled at 40°C to 40A / m 2 , keep the pH of the lithium-containing solution in the anode chamber at 3.5, and control the pH of the solution in the cathode chamber within the range of 4-5. After 14 hours of electrolysis, the concentration of metal ions in the cathode chamber is shown in the table below.
[0059]
Embodiment 3
[0061] Using the electrolytic cell mentioned above, the material of the anode is high-purity graphite, the material of the cathode is stainless steel, and the two ion exchange membranes are proton exchange membranes. The anode chamber is filled with 300ml of lithium-containing solution, and 2700mL is prepared externally for circulation replenishment. The cation composition is: Mg 2+ 95g / L, Li + 0.5g / L, Na + 5.4g / L, K + 3.2g / L and Ca 2+ 3g / L, the organic phase chamber contains tributyl phosphate and ClO - 4 300ml of supporting electrolyte with a concentration of 0.1mol / L HCl was filled into the cathode chamber. The solutions in the three chambers are respectively circulated through the pump, and the current density is controlled at 15A / m at 20°C 2 , keep the pH of the lithium-containing solution in the anode chamber at about 5, and the pH of the solution in the cathode chamber at about 4. After 30 hours of electrolysis, the concentration of metal ions in the cathode cham...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com