Propane dehydrogenation catalyst and preparation method thereof
A propane dehydrogenation and catalyst technology, which is applied in the direction of catalyst activation/preparation, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem that the performance of the catalyst is not particularly ideal, the stability needs to be further improved, Low reaction selectivity and other problems, to achieve the effect of improving anti-carbon deposition ability, ensuring thermal stability, and high dehydrogenation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
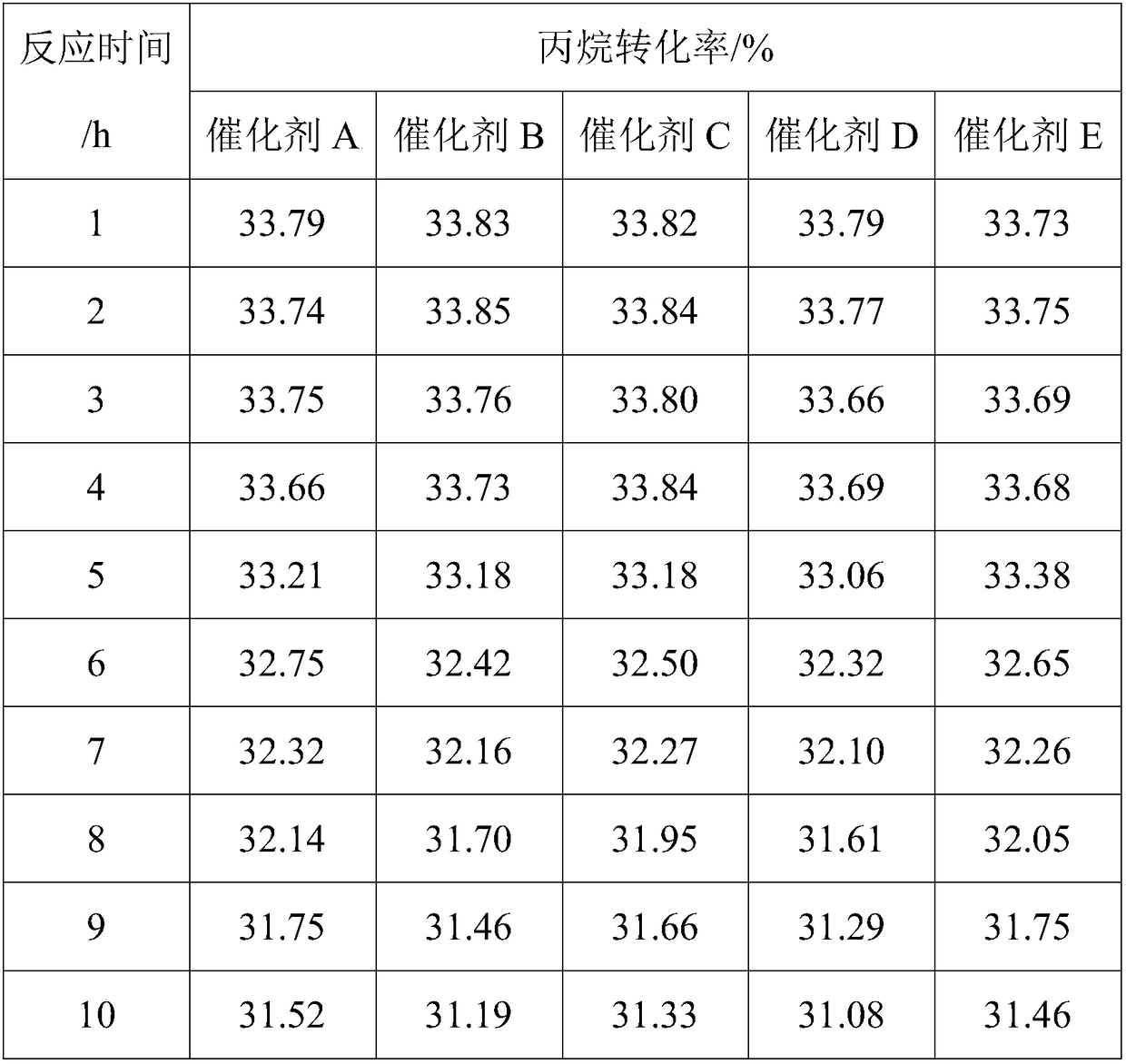
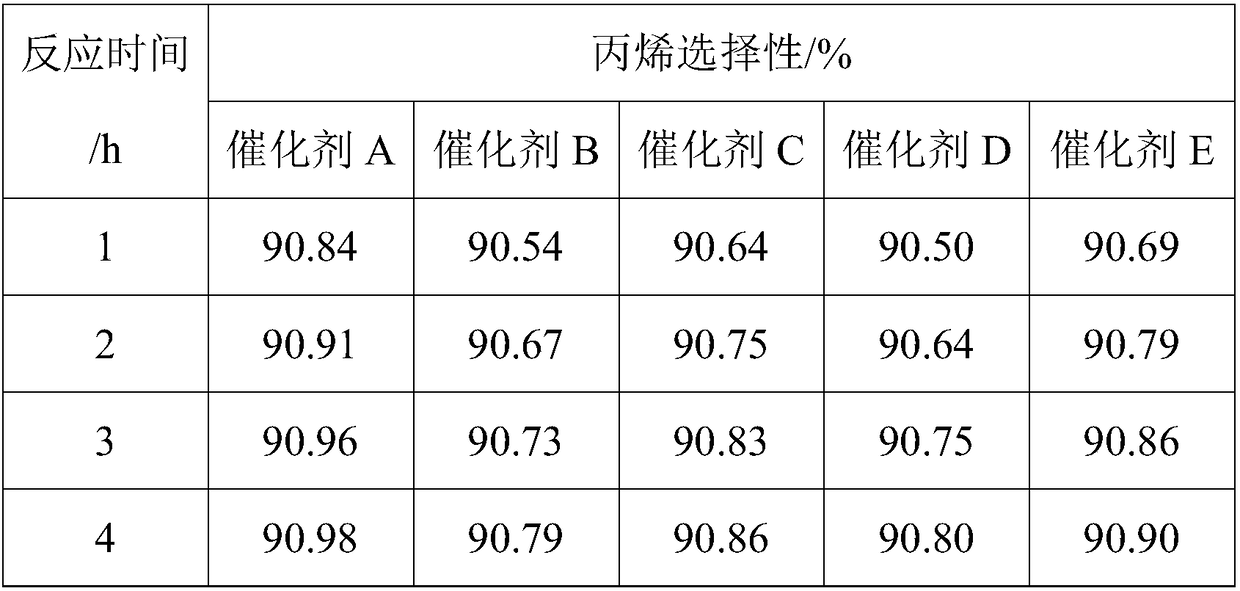
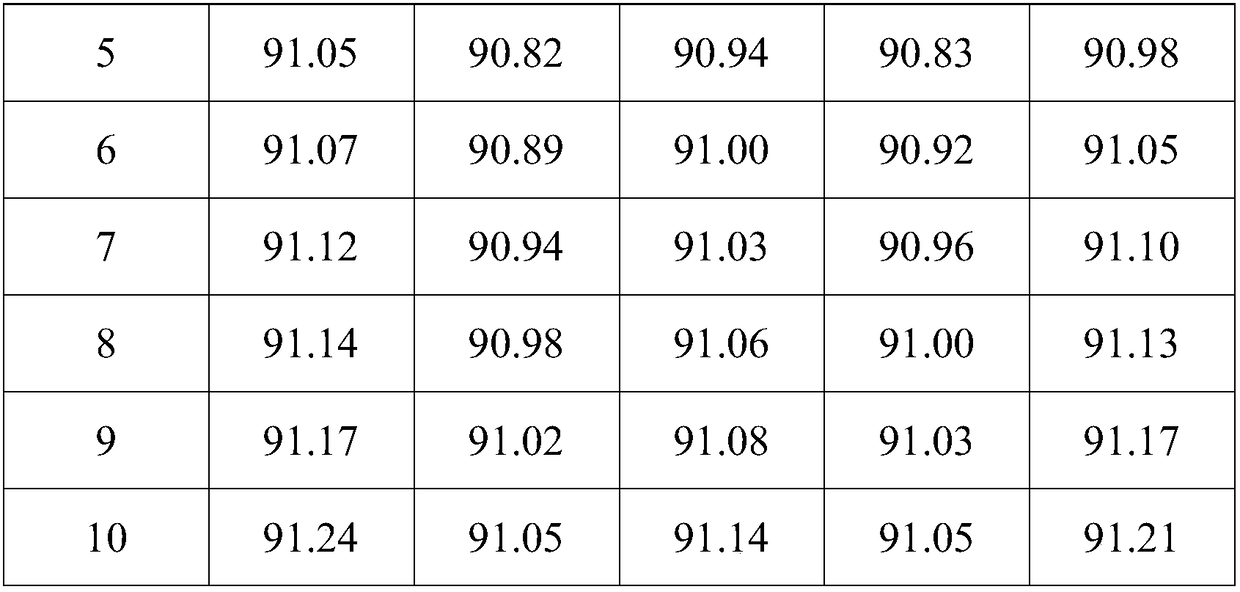
Examples
Embodiment 1
[0031] Catalyst preparation
[0032] (1) Weigh 35.290g Al(NO 3 ) 3 9H 2 O (molecular weight: 375) and 0.439g Ca (NO 3 ) 2 (Molecular weight: 164) was dissolved in 100ml deionized water to form a solution, and stirred for 1h; the solution was heated to 50°C, neutralized with 5wt% sodium carbonate solution, and the pH was controlled to be 9; after filtering and washing with deionized water, use 12mol / L nitric acid solution was acidified to pH 6, stirred to a sol-like state, and the carrier slurry was obtained. The slurry was taken with a 5mL syringe, fitted with a needle, and the slurry was added dropwise to the oily ammonia column. The sol shrank into a spherical gel, and the small The particle size of the ball is 1.4-1.6 mm. After aging for 2 hours, the gel ball is taken out, washed with deionized water, dried at 120°C for 12 hours, and calcined at 500°C for 8 hours to obtain a composite oxide carrier ball;
[0033] (2) Weigh 0.019g SnCl 2 2H2 O (molecular weight: 226) ...
Embodiment 2
[0038] Catalyst preparation
[0039] (1) Weigh 35.560g Al(NO 3 ) 3 9H 2 O and 0.366g Ca(NO 3 ) 2 Dissolve in 100ml deionized water to form a solution, and stir for 2 hours; heat the solution to 40°C, neutralize with 4wt% sodium carbonate solution, and control the pH to 9; filter and wash with deionized water, use 14mol / L nitric acid solution Acidify to pH 6, stir until it is in the form of a sol to obtain a carrier slurry, use a 5mL syringe to take the slurry, install a needle, add the slurry dropwise to the oily ammonia column, the sol shrinks into a spherical gel, and the particle size of the pellet is controlled to be 1.4 ~1.6mm, after aging for 2 hours, the gel beads were taken out, washed with deionized water, dried at 100°C for 24 hours, and calcined at 600°C for 6 hours to obtain composite oxide carrier beads;
[0040] (2) Weigh 0.029g SnCl 2 2H 2 O and 0.023g BiCl 3 Dissolve in 10.0ml of hydrochloric acid solution with a concentration of 1.0mol / L, make a soluti...
Embodiment 3
[0045] Catalyst preparation
[0046] (1) Weigh 35.330g Al(NO 3 ) 3 9H 2 O and 0.366g Ca(NO 3 ) 2 Dissolve in 100ml deionized water to form a solution, and stir for 1 hour; heat the solution to 60°C, neutralize with 6wt% sodium carbonate solution, and control the pH to 10; filter and wash with deionized water, use 11mol / L nitric acid solution Acidify to pH 6, stir until it is in the form of a sol to obtain a carrier slurry, use a 5mL syringe to take the slurry, install a needle, add the slurry dropwise to the oily ammonia column, the sol shrinks into a spherical gel, and the particle size of the pellet is controlled to be 1.4 ~1.6mm, after aging for 1 hour, take out the gel beads, wash with deionized water, dry at 150°C for 18 hours, and bake at 700°C for 8 hours to obtain composite oxide carrier beads;
[0047] (2) Weigh 0.019g SnCl 2 2H 2 O and 0.060 g BiCl 3 Dissolve in 10.0ml of hydrochloric acid solution with a concentration of 0.5mol / L, make a solution and stir ev...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com