Precious metal hydrodesulfurization catalyst as well as preparation method and application thereof
A hydrodesulfurization and precious metal technology, applied in the direction of catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, chemical instruments and methods, etc., can solve the problem of uneven distribution of active metal components, organic sulfide desorption Eliminate the problems of poor effect and poor thermal stability, and achieve the effects of good hydrodesulfurization effect, low price, improved dispersibility and anti-sintering ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
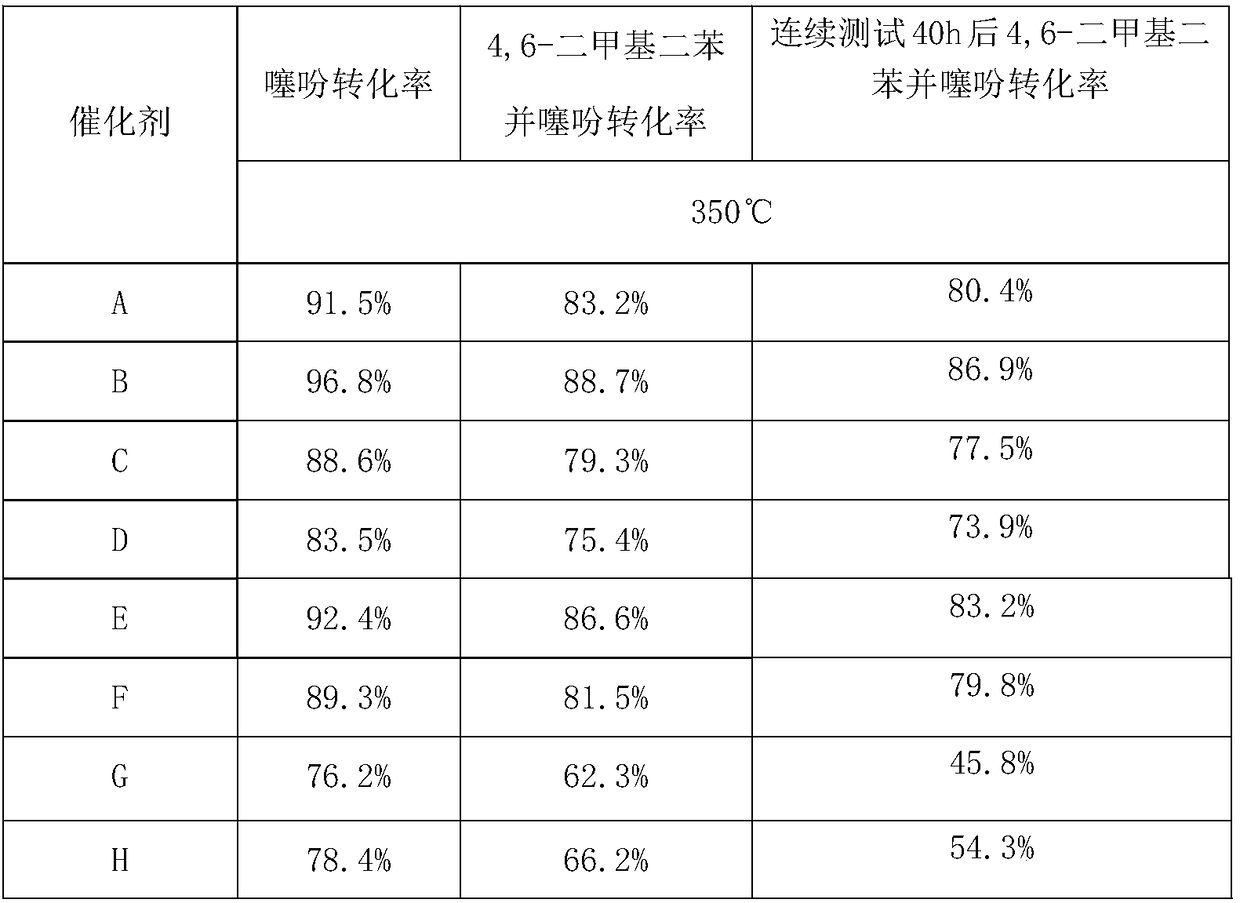
Examples
Embodiment 1
[0037] Preparation of cobalt-containing hydrotalcites by co-precipitation method: 6.4015g NaOH and 1.9875g anhydrous Na 2 CO 3 Dissolve in 200ml deionized water, stir for 20min, pour into a three-neck flask and continue stirring for 1h to obtain a precipitant solution; weigh 8.1852g Co(NO 3 ) 2 ·6H 2 O, 7.2116g Mg(NO 3 ) 2 ·6H 2 O and 7.0331g Al(NO 3 ) 3 9H 2 O and dissolved in 200ml deionized water, fully stirred and dissolved to obtain a mixed solution of cobalt salt, magnesium salt and aluminum salt; then use a peristaltic pump to slowly drop the mixed solution of cobalt salt, magnesium salt and aluminum salt Put it into the precipitant solution and stir vigorously. After the dropwise addition is completed, raise the temperature to 60°C, continue to stir for 1 hour, let it stand for 24 hours after the reaction is completed, centrifuge the reaction solution, collect the precipitate and wash it to pH = 7; then at 100°C After drying for 12 hours, CoMgAl-LDHs were obta...
Embodiment 2
[0040] Co-precipitation method to prepare cobalt-containing hydrotalcites: 6.3978g NaOH and 2.6523g anhydrous Na 2 CO 3 Dissolve in 200ml deionized water, stir for 20min, pour into a three-neck flask and continue stirring for 1h to obtain a precipitant solution; weigh 14.8745g Co(NO 3 ) 2 ·6H 2 O and 9.3783g Al(NO 3 ) 3 9H 2 O and be dissolved in 200ml deionized water, obtain the mixed solution of cobalt salt and aluminum salt after fully stirring and dissolving; Then with peristaltic pump, the mixed solution of cobalt salt and aluminum salt is slowly dripped in the precipitation agent solution with the speed of 6ml / min and Stir vigorously, after the dropwise addition is completed, raise the temperature to 60°C, continue to stir and react for 1h, after the completion of the reaction, let it stand for 24h, centrifuge the reaction solution, collect the precipitate and wash it to pH = 7; then dry at 100°C for 12h to obtain cobalt aluminum Hydrotalcite-like CoAl-LDHs.
[00...
Embodiment 3
[0043] Preparation of cobalt-containing hydrotalcites by co-precipitation method: 6.4017g NaOH and 3.1826g anhydrous Na 2 CO 3 Dissolve in 200ml deionized water, stir for 20min, pour into a three-necked flask and continue stirring for 1h to obtain a precipitant solution; weigh 4.4624g Co(NO 3 ) 2 ·6H 2 O and 5.6269g Al(NO 3 ) 3 9H 2 O and be dissolved in 200ml deionized water, obtain the mixed solution of cobalt salt and aluminum salt after fully stirring and dissolving; Then with peristaltic pump, the mixed solution of cobalt salt and aluminum salt is slowly dripped in the precipitation agent solution with the speed of 6ml / min and Stir vigorously, after the dropwise addition is completed, raise the temperature to 60°C, continue to stir and react for 1h, after the completion of the reaction, let it stand for 24h, centrifuge the reaction solution, collect the precipitate and wash it to pH = 7; then dry at 100°C for 12h to obtain cobalt aluminum Hydrotalcite CoAl-LDHs.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com