Novel valganciclovir crystal form
A technology of crystal form and acetone, which is applied in the field of preparation of raw materials, can solve the problems that valganciclovir is easy to absorb moisture, achieve good anti-hygroscopic performance, avoid crystal form changes and increase the effect of related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The first step 18g valganciclovir crude product is dissolved in 100ml ethanol-acetone mixed solvent, and in described mixed solvent, the volume ratio of ethanol and acetone is 1:0.3.
[0025] Step 2: At room temperature, add 1.45 g of activated carbon to the solution in Step 1, stir for 1 hour, and filter;
[0026] In the third step, under stirring, the temperature of the filtrate in the second step was lowered to -12°C and kept, and 17ml of acetone was slowly added dropwise at a rate of 0.90ml / min. A white solid was precipitated, and the stirring was continued for 3-5 hours. Stirring speed is 170 rpm;
[0027] The fourth step is to filter, and the filter cake is washed 3 times with absolute ethanol, each dosage is 8ml, the filter cake is spread out, and vacuum-dried at 40°C.
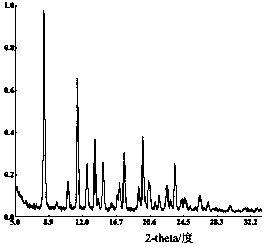
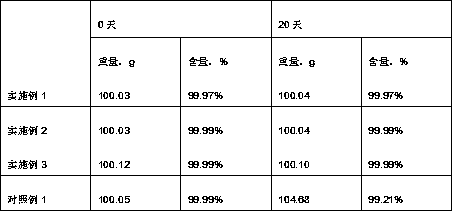
[0028] Obtain crystal formation described in the present invention, its X-ray powder diffraction figure sees attached figure 1 , with a melting point of 280.63°C, a purity of 99.97% detected by...
Embodiment 2
[0030] The first step 26g valganciclovir crude product is dissolved in ethanol-acetone mixed solvent, and in described mixed solvent, the volume ratio of ethanol and acetone is 1:0.6.
[0031] Step 2: At room temperature, add 2.6 g of activated carbon to the solution in Step 1, stir for 1 hour, and filter;
[0032] In the third step, under stirring, the temperature of the filtrate in the second step is lowered to -5°C and maintained, and 25ml of acetone is slowly added dropwise at a rate of 1.5ml / min. A white solid is precipitated, and the stirring is continued for 3-5 hours , the stirring speed is 190 rpm;
[0033] The fourth step is to filter, and the filter cake is washed 3 times with absolute ethanol, each dosage is 10ml, the filter cake is spread out, and vacuum-dried at 40°C.
[0034] Obtain crystal formation described in the present invention, its X-ray powder diffraction figure sees attached figure 1 , with a melting point of 281.01°C, a purity of 99.99% detected by ...
Embodiment 3
[0036] The first step 22g valganciclovir crude product is dissolved in ethanol-acetone mixed solvent, in described mixed solvent, the volume ratio of ethanol and acetone is 1:0.45;
[0037] Step 2: At room temperature, add 2 g of activated carbon to the solution in Step 1, stir for 1 hour, and filter;
[0038] In the third step, under stirring, the temperature of the filtrate in the second step was lowered to -7°C and kept, and 21ml of acetone was slowly added dropwise at a rate of 1.2ml / min. A white solid was precipitated, and the stirring was continued for 3-5 hours. Stirring speed is 180 rpm;
[0039] The fourth step is to filter, and the filter cake is washed 3 times with absolute ethanol, each dosage is 9ml, the filter cake is spread out, and vacuum-dried at 40°C.
[0040] Obtain crystal formation described in the present invention, its X-ray powder diffraction figure sees attached figure 1 , melting point 280.92°C, HPLC purity of 99.99%, yield of 92.14%, particle size ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle size distribution | aaaaa | aaaaa |
| particle size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com