Method for continuously preparing multi-metal oxide pore structure catalyst for carbon nanotube
A multi-metal oxide and structural catalyst technology, applied in the direction of metal/metal oxide/metal hydroxide catalysts, carbon nanotubes, multi-walled carbon nanotubes, etc., can solve the problem of porous structure catalysts without catalytic carbon nanotubes To achieve the effect of uniform distribution of active points, uniform catalyst particles and sufficient reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
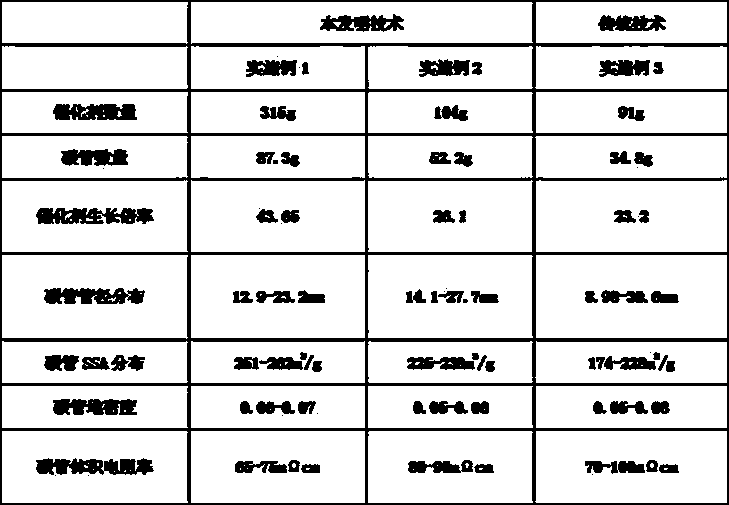
Embodiment 1
[0037] Example 1: In this example, the method of the present invention is used to prepare an iron-based catalyst. The formula is as follows:
[0038] A active metal salt solution
[0039] 1350g of aluminum nitrate nonahydrate, 610g of iron nitrate nonahydrate, 4000g of deionized water; add 4000g of deionized water to a 10L beaker, add 1350g of aluminum nitrate nonahydrate and 610g of iron nitrate nonahydrate, and heat the heating mantle to 50°C- 55°C, stir with a glass rod until it dissolves and set aside.
[0040] B alkaline solution of inert metal salt
[0041] Ammonium heptamolybdate tetrahydrate 40g, ammonium carbonate 550g, 17% ammonia water 190g, deionized water 3500g.
[0042] Preparation method: add 3500g of deionized water to a 10L beaker, add 550g of ammonium carbonate and 40g of ammonium heptamolybdate tetrahydrate successively, raise the temperature of the heating mantle to 40°C-50°C, stir with a glass rod until it dissolves, then add 190g 17% ammonia water, st...
Embodiment 2
[0046] Example 2 In this example, the present invention is used to prepare a nickel-based catalyst. The formula is as follows:
[0047] A active metal salt solution
[0048] Nickel nitrate hexahydrate 275g,
[0049] Magnesium nitrate hexahydrate 230g,
[0050] Deionized water 790g.
[0051] Add 790g of deionized water to a 2L beaker, add 275g of nickel nitrate hexahydrate and 230g of magnesium nitrate hexahydrate successively, raise the temperature of the heating mantle to 50°C-55°C, stir with a glass rod until it dissolves and set aside.
[0052] B alkaline solution of inert metal salt
[0053] Ammonium heptamolybdate tetrahydrate 19g,
[0054] Ammonium carbonate 350g,
[0055] 120g of 17% ammonia water,
[0056] Deionized water 680g.
[0057] Preparation method: Add 680g of deionized water to a 2L beaker, then add 350g of ammonium carbonate and 19g of ammonium heptamolybdate tetrahydrate, heat up the heating mantle to 40°C-50°C, stir with a glass rod until it dissolv...
Embodiment 3
[0061] Example 3: In this example, a cobalt-based catalyst was prepared by using traditional manual controlled dropwise addition. The formula is as follows:
[0062] A active metal salt solution
[0063] Aluminum nitrate nonahydrate 675g, cobalt nitrate hexahydrate 458g, deionized water 1900g.
[0064] Preparation method: Add 1900g of deionized water to a 5L beaker, add 675g of aluminum nitrate nonahydrate and 458g of cobalt nitrate hexahydrate successively, raise the temperature of the heating mantle to 55°C-60°C, stir with a glass rod until it dissolves and set aside.
[0065] B alkaline solution of inert metal salt
[0066] Ammonium heptamolybdate tetrahydrate 18g, ammonium carbonate 300g, 17% ammonia water 100g, deionized water 1800g. Preparation method: add 1800g of deionized water to a 5L beaker, add 300g of ammonium carbonate and 18g of ammonium heptamolybdate tetrahydrate, heat up the heating mantle to 55°C-60°C, stir with a glass rod until it dissolves, then add 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com