Probe molecule based on 1,8-naphthalimide derivative, preparation method and application
A technology of naphthalimide and probe molecules, which is applied in chemical instruments and methods, analytical materials, material excitation analysis, etc., can solve the problems of strict test conditions, complex molecular structure, cumbersome synthesis process of fluorescent probes, etc., and achieve anti- Strong interference ability, simple synthesis method, high sensitivity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
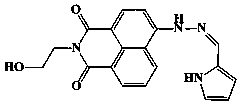
[0031] This embodiment provides a Cu based on 1,8-naphthalimide derivatives 2+ Probe molecule, its synthetic route is as follows:
[0032] .
[0033] The concrete steps of synthetic method are as follows:
[0034] 1. Add 10mmol 4-bromo-1,8-naphthalene dicarboxylic anhydride to 100ml ethanol, then add dropwise 12mmol ethanolamine dissolved in 10ml ethanol, stir and reflux the mixture at 80°C for 4 hours, after the reaction, The reaction solution was cooled to room temperature, filtered and washed with water to obtain a white solid (4-bromo-N-2-aminoethanol-1,8-naphthalimide), which was designated as product Ⅰ.
[0035] 2. Add 5 mmol of white solid I and 10 mmol of hydrazine hydrate obtained in step 1 into 150 ml of ethylene glycol monomethyl ether, stir and reflux at 125°C for 10 hours, after the reaction is completed, cool the reaction solution to room temperature, and dissolve the reaction solution Pour into water, filter, wash with water, and dry to obtain a yellow soli...
Embodiment 2
[0040] In this embodiment, the probe molecules prepared in Example 1 are applied to detect Cu 2+ , its properties and experimental methods are as follows:
[0041] 1. Separately prepare Ag with deionized water + , Ca 2+ , Co 2+ , Cu 2+ , Fe 2+ , Fe 3+ , K + , Li + , Mg 2+ , Na + , Ni 3 + , and Zn 2+ Aqueous solutions (24 μM) of various metal salts.
[0042] 2. Weigh 0.0035 g of the probe molecule prepared in Example 1 of the present invention, and dissolve it in 100 ml of acetonitrile (CH 3 CN) prepared in 1.0×10 -4 M's solution. Dilute the acetonitrile solution of the probe molecule to 10 μM of the probe molecule CH 3 CN-H 2 O (V / V=3:7, pH=7.0) solution for use.
[0043] 3. Add the probe molecule solutions provided in step (2) to the aqueous solutions of various metal salts provided in step (1), and use 442 nm as the excitation wavelength to measure their fluorescence emission wavelengths and intensities. For the results, see the attached figure 2 . fig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com