Double-pillararene mercury ion fluorescence sensor as well as preparation and application thereof
A fluorescent sensor and mercury ion technology, applied in the field of ion detection, can solve the problems of expensive instruments, restricting the use of detection methods, complex operations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, the synthesis of mercury ion sensor QS
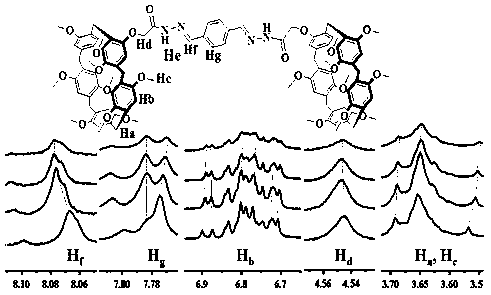
[0043] Weigh 0.48 g (0.6 mmol) of amidated column[5]arene (for the synthesis of amidated column[5]arene, see literature [G. F. Huo, Y. Han, J. Sun, C. G. Yan, J. Incl. Phenom. Macrocycl. Chem .,2016, 86,231-240.]) into a 100mL round bottom flask, add 50mL ethanol as solvent, slowly add 0.04g (0.3mmol) terephthalaldehyde and 0.5mL (8.8mmol) glacial acetic acid under stirring Catalyst, stirred at room temperature for 30min, heated to reflux at 80°C on an oil bath for 16h. Heat pumping after the reaction stops. The resulting solid was washed with ethanol three to five times, and then dried in a vacuum oven to obtain 0.81 g of a light yellow solid, which is the mercury ion sensor QS, with a yield of 80%.
[0044] (m.p. 140-143°C), 1 HNNR (600 MHZ, CDCl 3 ), δ 9.85-9.82 (d, J =18 Hz 2H,NH), δ 8.23-8.21 (d, J =12 Hz, 2H, N=CH), δ 7.82 (s, 3H, ArH), δ 6.78-6.58 (m,21H, ArH), δ 4.47 (s, 4H, OCH 2 CO), δ 3.87-3.45 (m...
Embodiment 2
[0047] Embodiment 2, sensor QS fluorescent recognition Hg 2+
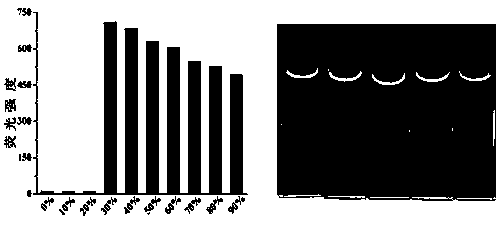
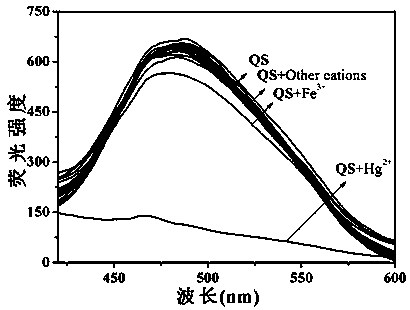
[0048] Pipette 0.5 mL of QS (2×10 -4 mol L -1 ) DMSO solution in a series of 10 mL colorimetric tubes, and then add Hg 2+ , Ca 2+ , Mg 2+ , Ni 2+ , Cu 2+ , Cr 2+ , Cd 2+ , Pb 2+ , Ag + , Zn 2+ , Fe 3+ , Ba 2+ ,Co 2+ , La 3+ , Eu 2+ , Tb 2+ DMSO-H 2 O solution, if the fluorescence of QS is quenched, it means that Hg was added 2+ , if the fluorescence of QS does not change, it means that the addition of Hg is not 2+ .
Embodiment 3
[0049] Embodiment 3, preparation and application of mercury ion responsive film
[0050] Preparation of mercury ion-responsive films: glass plates were immersed in high concentration (200 µM) of QS in DMSO-H 2 O (water volume is 30%) solution, take it out and dry it in the air to form a QS film. The film exhibits yellow-green fluorescence under 365 nm UV light.
[0051] Hg 2+ Application of safety display materials: Dip an appropriate amount of Hg with a fine brush 2+ DMSO-H 2 O solution (C = 0.1mol / L), write a word on the gel film, such as "Hg", under a 365 nm ultraviolet lamp, it can be observed that the fluorescence of the writing part of the film is quenched, and the written content is highlighted (see Figure 7 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com