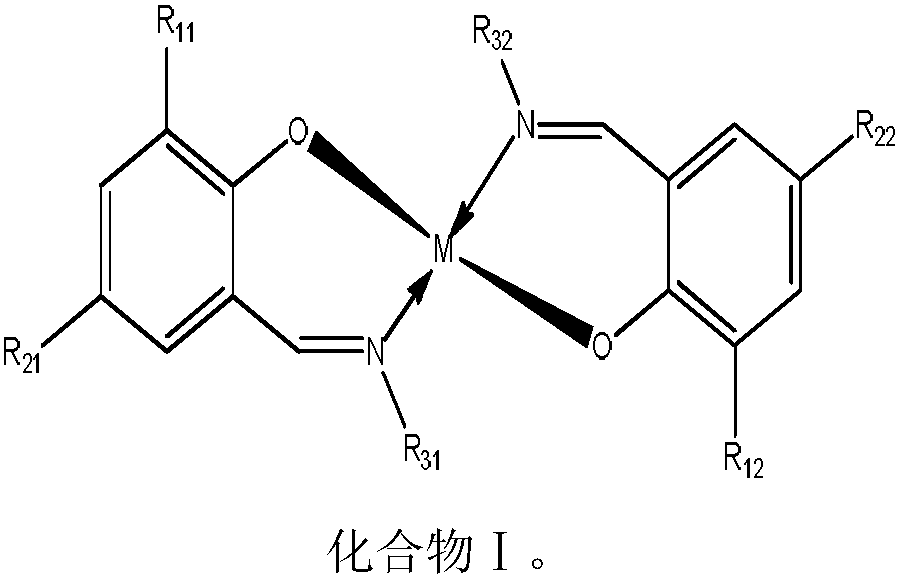
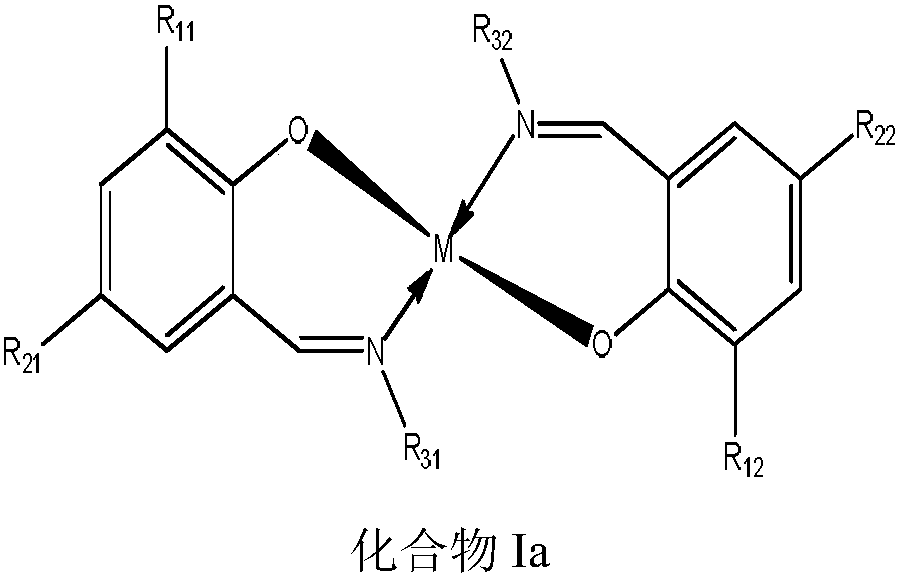
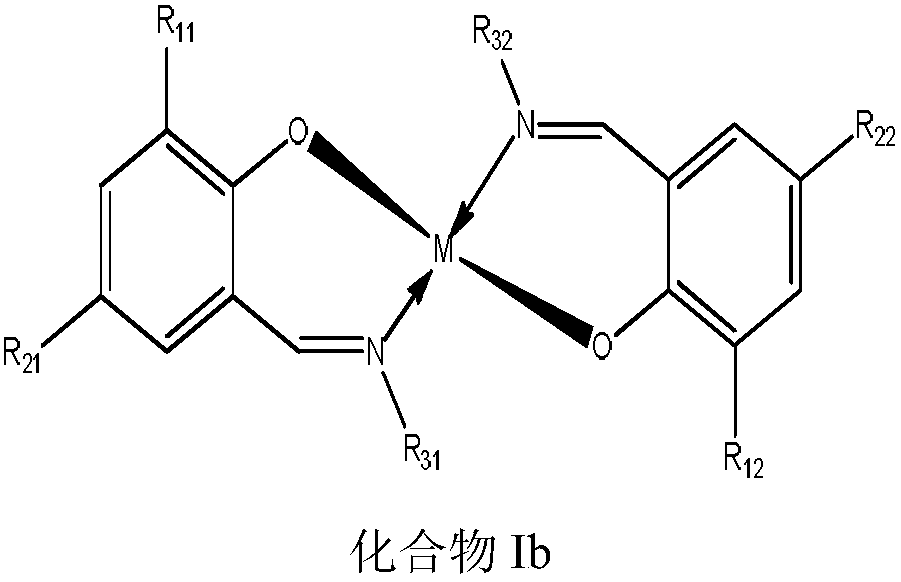
Method for preparing canthaxanthin through oxidation of beta-carotene
A technology of carotene and canthaxanthin, which is applied in the direction of organic chemistry, can solve the problems of large usage, environmental pollution, and low yield, and achieve the effects of increasing yield, reducing resource consumption, and eliminating emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Dissolve 0.1mol β-carotene in 1.8L of dichloromethane in a 2L reactor, then add 0.001mol of catalyst (compound Ia) and 0.01mol of iodine, and cool the reaction solution to -20°C in an airtight state , feed oxygen under stirring, control the reaction gauge pressure to be 0.1MPa, control the reaction temperature to be -20°C, stir the reaction until the reaction solution β-carotene is completely converted, detect the content of cantharidin in the reaction solution, and calculate the cantharidin The yellow yield was 75.53%.
[0063]
[0064] (R 11 = H R 12 = H R 21 = H R 22 = H R 31 =CH 2 CH 2 OH R 32 =CH 2 CH 2 OH M=Cu)
Embodiment 2
[0066] In a 2L reactor, 0.1mol β-carotene was dissolved in 1.5L of chloroform, then 0.01mol of catalyst (compound Ib) and 0.01mol of potassium iodide were added, and the reaction solution was cooled to 0° C. in an airtight state. Add the hydrogen peroxide (30%W) that contains 2mol hydrogen peroxide under the situation of stirring, control reaction temperature and be 0 ℃, stir reaction until reaction solution β-carotene completely transforms, detect the content of cantharidin in the reaction solution, calculate cantharidin The yellow yield was 80.07%.
[0067]
[0068] (R 11 = H R 12 = H R 21 =SO 3 H R 22 = SO 3 H R 31 =CH 2 CH 2 SO 3 HR 32 =CH 2 CH 2 SO 3 H M = Cu)
Embodiment 3
[0070] Dissolve 0.1mol β-carotene in 1.5L tetrachloromethane in a 2L reactor, then add 0.01mol catalyst (compound Ic) and 0.01mol potassium iodide, and heat the reaction solution to 60°C in an airtight state , feed air under stirring, control the reaction gauge pressure to be 0.1MPa, control the reaction temperature to be 60°C, stir the reaction until the reaction solution β-carotene is completely converted, detect the content of cantharidin in the reaction solution, and calculate cantharidin The yield is 60.37%.
[0071]
[0072] (R 11 = H R 12 = H R 21 = H R 22 = H R 31 =CH 2 CH 2 OH R 32 =CH 2 CH 2 OH M=Mn)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com