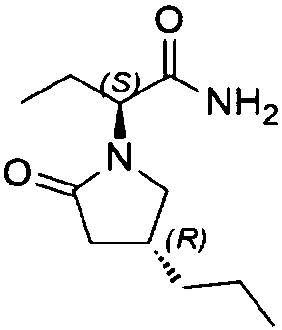
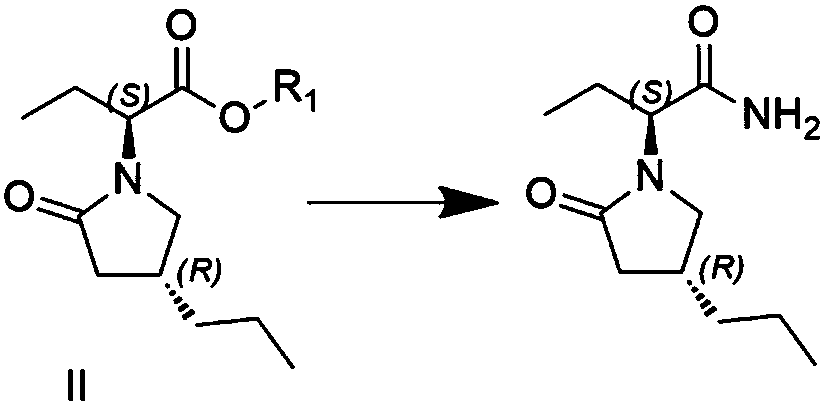
Preparation method of high chiral purity lactam intermediate and brivaracetam
A technology for intermediates and lactams, which is applied to the preparation of high chiral purity lactam intermediates, the preparation field of brivaracetam, can solve the problems of complicated steps, high equipment requirements, difficult purification and the like, and achieves simple separation process, Simple and safe operation, improve the effect of selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
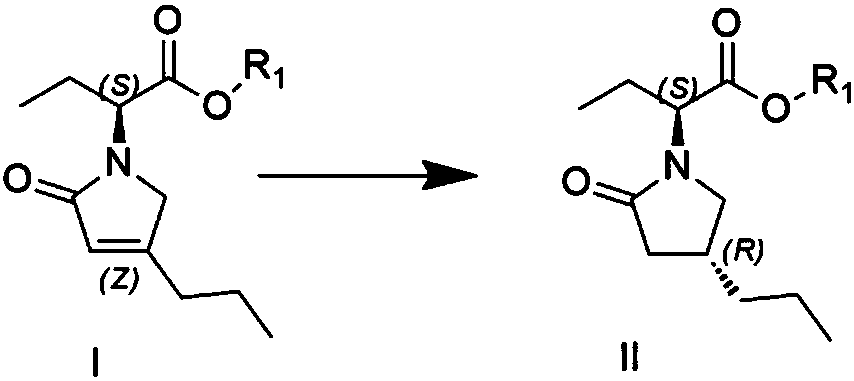
[0033] Preparation of compound formula II
[0034] Add citric acid monohydrate (0.27g, 0.0013mol) in 250ml four-necked reaction flask, water 30ml, methanol 15ml dissolves, add 10% palladium carbon 0.15g, stir, add (S-2-(4-propyl-1 , 5-dihydropyrrol-2-one) butyric acid methyl ester) 3g0.013mol, temperature control -20 ℃, hydrogen replacement, hydrogen pressure is 1bar, stirring reaction, after 20 hours of reaction, TLC control until the raw materials disappear completely, Stop the reaction, filter, spin off the organic solvent at 35°C, add 50ml*2 methyl acetate for extraction, dry over anhydrous sodium sulfate, and spin dry to obtain 2.7g (0.012mol), yield 89%, HPLC 97.65%, de% 98.6 %.
Embodiment 2
[0036] Preparation of compound formula II
[0037] Add malonic acid (1.35g, 0.013mol) in 250ml four-necked reaction flask, water 30ml, ethanol 30ml dissolve, add 0.3g of 10% palladium calcium carbonate, stir, add (S-2-(4-propyl-1 , 5-dihydropyrrol-2-one) butyric acid methyl ester) 3g, 0.013mol, temperature control -20 ℃, hydrogen replacement, hydrogen pressure is 1 bar, stirring reaction, after 20 hours of reaction, control in TLC until the raw materials disappear completely , stop the reaction, filter, spin off the organic solvent at 35°C, add 50ml*2 methyl acetate for extraction, dry over anhydrous sodium sulfate, and spin dry to obtain 2.5g (0.011mol), yield 85%, HPLC 96.3%, de% 98.1%
Embodiment 3
[0039] Preparation of compound formula II
[0040] Add formic acid (1g, 0.022mol) to a 250ml reactor, dissolve in 50ml of water and 25ml of acetonitrile, add 0.25g of 5% platinum on carbon, stir, add (S-2-(4-propyl-1,5-dihydropyrrole- 2-keto) methyl butyrate) 5g, 0.022mol, temperature control 20°C, hydrogen replacement, hydrogen pressure 4bar, stirring reaction, after 30 hours of reaction, TLC control until the raw materials disappeared completely, stop the reaction, filter, 35°C Spin off the organic solvent, add 50ml*2 methyl acetate for extraction, dry over anhydrous sodium sulfate, and spin dry to obtain 4.6g (0.020mol), yield 90.9%, HPLC 98.21%, de% 98.6%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com