A method for co-producing methylbenzoic acid, methylbenzoyl chloride and phthaloyl chloride
A technology of toluoyl chloride, phthaloyl chloride, and toluic acid is applied in chemical instruments and methods, separation/purification of carboxylic acid compounds, preparation of carboxylate salts, etc., and can solve the problem of low yield of target products, Problems such as large amount of solid waste and single product structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
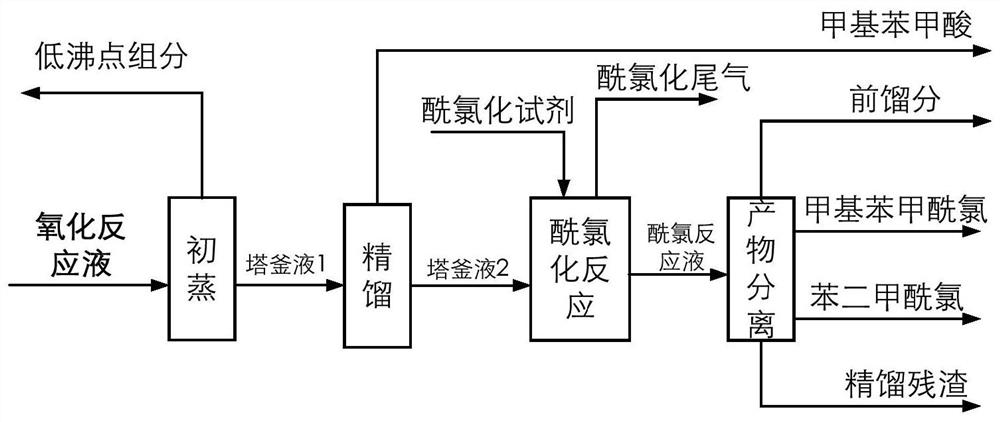
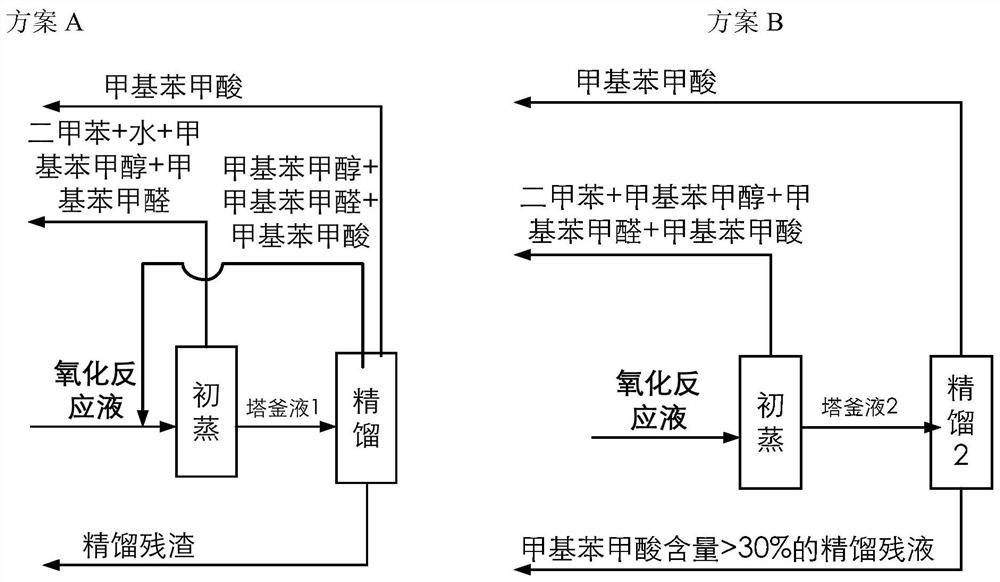
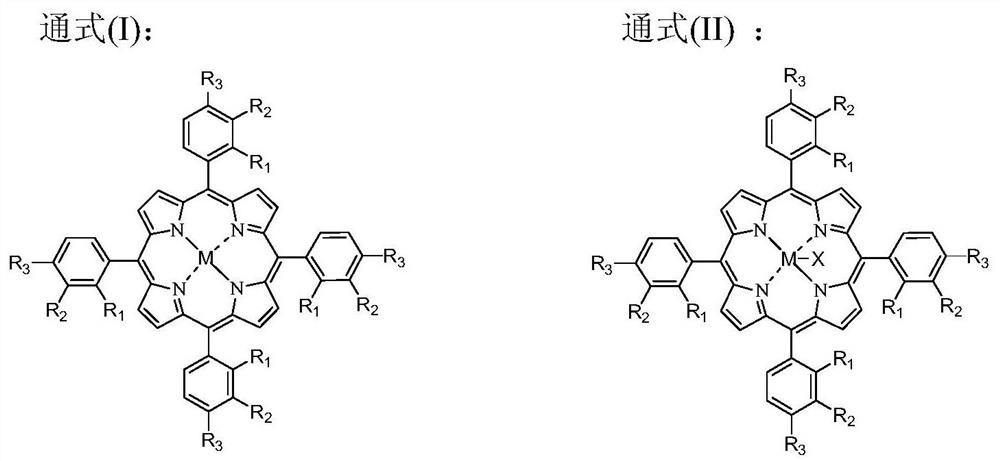
[0100] The catalyst that dissolves in the fresh p-xylene that adds in the oxidation reactor is cobalt naphthenate, metal phthalocyanine (R with general formula (IV) structure 1 =CH 3 CH 2 , R 2 =H, M=Mn), the metalloporphyrin (R 1 =R 2 = H, R 3 =CH 3 , M=Cu) mixture, the total concentration is 75ppm, the reaction temperature is 180°C, the reaction pressure is 2MPa, the oxygen-containing gas is the oxygen-enriched air of 24% oxygen concentration, the residence time of the reactants in the oxidation reactor is adjusted to make the The conversion rate of xylene was 42%, and the oxidation reaction solution was obtained. At this time, the mass percentage of each component in the reaction solution obtained by HPLC analysis is listed in Table 1. In the initial distillation tower, the boiling point in the oxidation reaction liquid is lower than all components of p-methylbenzyl alcohol for rectification separation, and when the p-methylbenzyl alcohol content reaches 0.06wt% in th...
Embodiment 2
[0103] The catalyst dissolved in fresh p-xylene fed to the oxidation reactor was MnO 2 And cobalt acetylacetonate, total concentration is 350ppm, and reaction temperature is 150 ℃, and reaction pressure is 1MPa, oxygen-containing gas concentration 21%, operation process is identical with embodiment 1, and difference is that the conversion rate control of p-xylene is 50% , the mass percent content of p-methylbenzyl alcohol in the initial distillation tower still liquid is controlled to be 0.07%. The mass percent composition of each component that HPLC analysis obtains in the reaction solution is listed in Table 1, and the quality, composition and the content of methylbenzoic acid in the first distillation column tower bottom liquid that obtains are listed in the table 2.
[0104] The liquid in the first distillation tower was rectified to obtain 889.4 g of p-toluic acid product with a purity of 99.0% at the top of the tower, and 1226.0 g of a high-boiling liquid in the bottom ...
Embodiment 3
[0106] Add the catalyst dissolved in the fresh p-xylene in the oxidation reactor to be N-hydroxyphthalimide, cobalt isooctanoate, metal phthalocyanine (R with general formula (IV) structure 1 =OH, R 2 =H, M=Ru) and metalloporphyrins (R 1 =R 3 = H, R 2 =OH,M 1 = M 2 =Mn) mixture, the total concentration is 10000ppm, the reaction temperature is 116 ° C, the reaction pressure is 0.2MPa, the oxygen-containing gas is pure oxygen, the operation process is the same as in Example 1, and the difference is that the conversion rate of p-xylene is controlled as 60%, and the mass percent content of p-methylbenzyl alcohol in the bottom liquid of the primary distillation tower is controlled to be 0.10%. The mass percent composition of each component that HPLC analysis obtains in the reaction solution is listed in Table 1, and the quality, composition and the content of methylbenzoic acid in the first distillation column tower bottom liquid that obtains are listed in the table 2.
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com