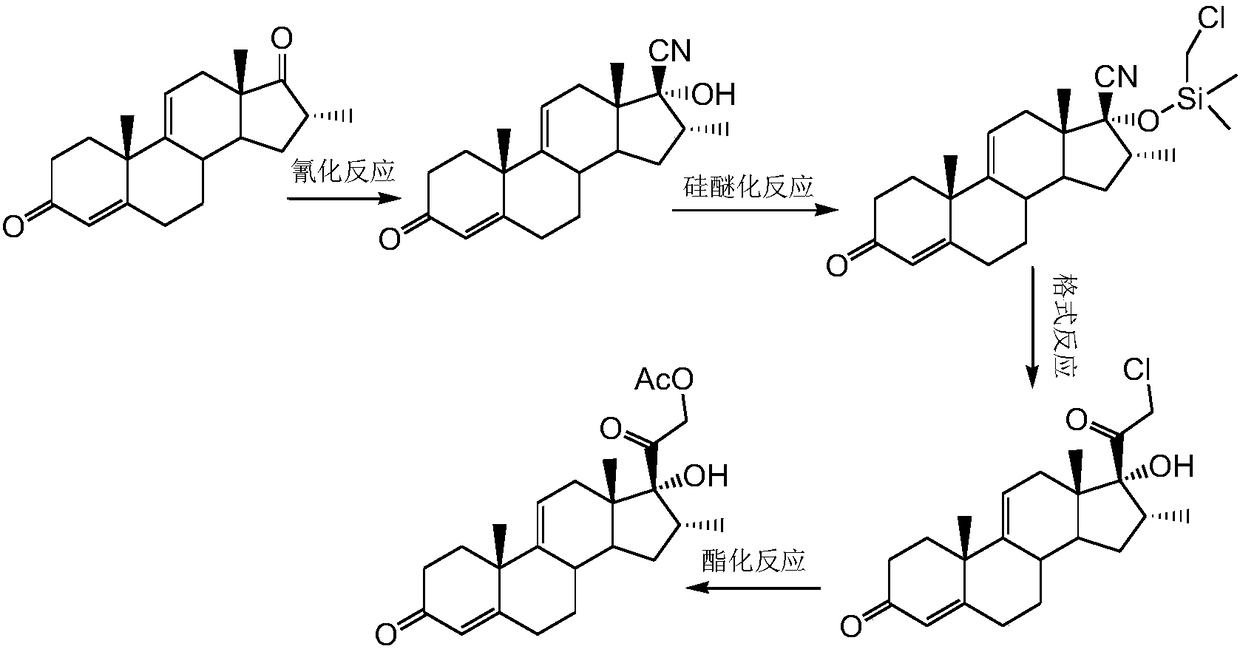
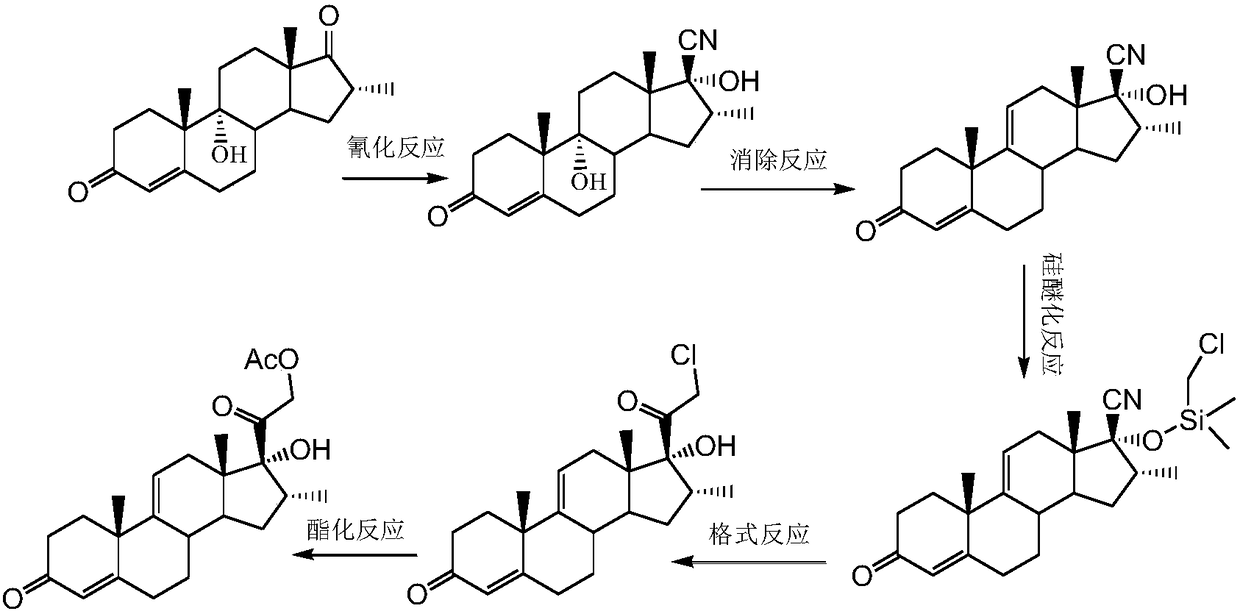
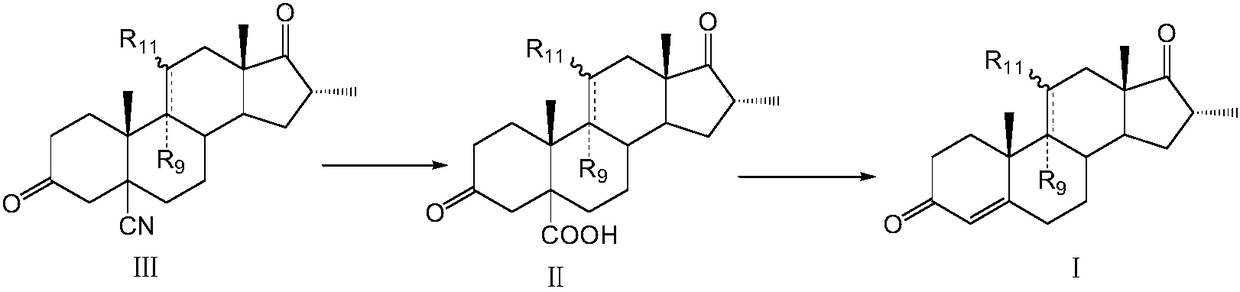
Method for preparing dexamethasone intermediate
A technology for dexamethasone and intermediates, which is applied in the field of preparation of steroid compounds, can solve the problems of low overall yield and high cost, and achieve the effects of improving balanced yield, benefiting environmental protection, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of 16α-methyl-4,9(11)-diene-3,17-dione
[0027] Hydrolysis reaction
[0028] At room temperature, add 400ml ethanol to a clean and dry 1000ml three-necked round bottom flask equipped with a thermometer and mechanical stirring, add 25g potassium hydroxide under stirring, stir to clear, add 50g 16α-methyl-5-cyano-9( 11)-ene-3,17-dione, the system immediately dissolves and turns into a brownish-red color. The temperature is raised to 40°C and kept for 1.5h. TLC control reaction is complete (developing solvent: acetone / petroleum ether=1:2), down to 10~15℃, start to add 15% hydrochloric acid dropwise to the system PH=4~5. At this time, a small amount of solid precipitates out of the reaction system. Concentrate under reduced pressure at 45~50℃ until there is no solvent. Add 500ml of water to stir and increase to 45℃. The salt was boiled for 1 h, filtered with suction, the filter cake was rinsed with water to neutrality, and the solid was dried at 50°C to o...
Embodiment 2
[0031] Example 2: Preparation of 16α-methyl-4,9(11)-diene-3,17-dione
[0032] Hydrolysis reaction
[0033] At room temperature, add 400ml methanol to a clean and dry 1000ml three-necked round bottom flask equipped with a thermometer and mechanical stirring. Add 25g sodium hydroxide while stirring. Stir to dissolve and add 50g 16α-methyl-5-cyano-9( 11)-En-3,17-dione, the system immediately dissolves and turns to brownish red. Raise it to 60℃ and keep it for 1h. TLC control reaction is complete (developing solvent: acetone / petroleum ether=1:2), down to 10 ~15℃, start to add 15% hydrochloric acid dropwise to the system PH=4~5. At this time, a small amount of solids are precipitated in the reaction system. Concentrate under reduced pressure at 45~50℃ until there is no solvent. Add 500ml of water to stir to disperse and boil at 45℃. Desalting for 1 h, suction filtration, rinse the filter cake with water to neutrality, and dry the solid at 50°C to obtain 48.5 g of hydrolysate, yield: 97...
Embodiment 3
[0036] Example 3: Preparation of 16α-methyl-4,9(11)-diene-3,17-dione
[0037] Hydrolysis reaction
[0038] At room temperature, add 400ml of acetone to a clean and dry 1000ml three-necked round bottom flask equipped with a thermometer and mechanical stirring, add 25g potassium hydroxide under stirring, stir to dissolve, add 50g 16α-methyl-5-cyano-9( 11)-En-3,17-dione, the system immediately dissolves and turns into a brownish-red color. The temperature is raised to 30°C for 3 hours, and the reaction is completed under TLC control (developing solvent: acetone / petroleum ether = 1:2), down to 10 ~15℃, start to add 15% hydrochloric acid dropwise to the system PH=4~5. At this time, a small amount of solids are precipitated in the reaction system. Concentrate under reduced pressure at 45~50℃ until there is no solvent. Add 500ml of water to stir to disperse and boil at 45℃. Desalting for 1 h, suction filtration, rinse the filter cake with water to neutrality, and dry the solid at 50°C to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com