Application of 1,8-Naphthalimide Derivatives as Substrates in maldi-ms
A technology of naphthalene dicarboximide and derivatives, applied in the field of mass spectrometry analysis, can solve the problems of increasing the complexity of the experiment, affecting the properties of the analyte, etc., and achieves the effects of wide range of use, strong repeatability and less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
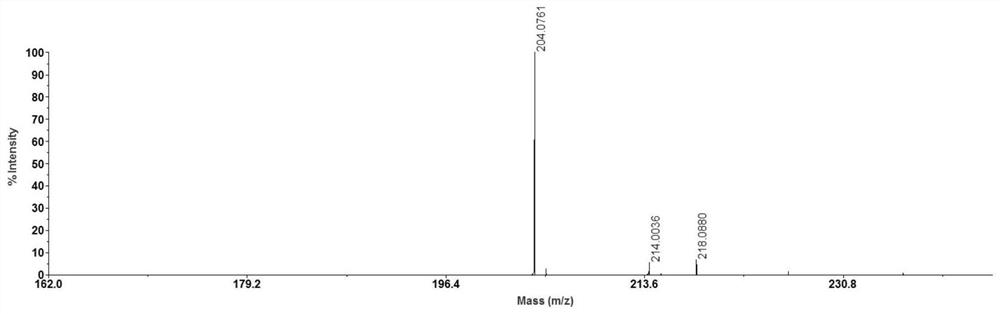
[0029] Weigh 1 mg of N-butyl-4-hydroxy-1,8-naphthalimide and dissolve it in 1 ml of a mixed solution of 50% acetonitrile and water containing 0.2% trifluoroacetic acid, sonicate for 5 minutes, and collect the overgrowth after centrifugation The membrane supernatant was used for later use. Weigh 1 mg of acetyl-L-carnitine hydrochloride and dissolve it in 1 ml of methanol, and sonicate for 5 minutes to obtain a clear solution of acetyl-L-carnitine hydrochloride. Dissolve 5 μL of the solution in 450 μL of methanol and dilute to 10 μg / ml for later use. Take 0.5 μL of N-butyl-4-hydroxy-1,8-naphthalimide solution on a clean MALDI target plate, place it in the air and wait for the solvent to evaporate naturally for MALDI-MS analysis. The results are shown in the attached figure 1 shown.
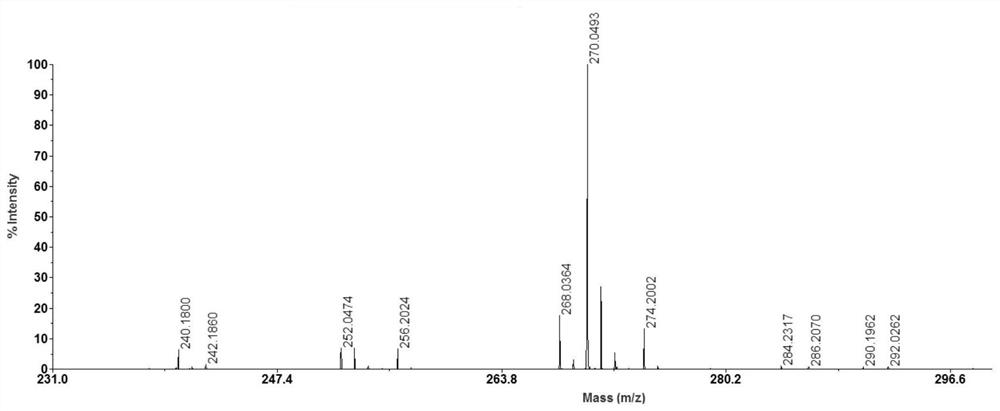
[0030] After N-butyl-4-hydroxy-1,8-naphthalimide solution was mixed with 10 μg / ml acetyl-L-carnitine hydrochloride solution in equal volumes, take 0.5 μL of the mixed solution on a clean MALDI targ...
Embodiment 2
[0032] Weigh 1 mg of N-butyl-4-hydroxy-1,8-naphthalimide and dissolve it in 1 ml of a mixed solution of 50% acetonitrile and water containing 0.2% trifluoroacetic acid, sonicate for 5 minutes, and collect the overgrowth after centrifugation The membrane supernatant was used for later use. Dissolve 500 μg of Aβ35-40 in 500 μl of methanol and sonicate for 5 minutes to obtain a clear Aβ35-40 solution. Dissolve 10 μL of this solution in 90 μL of methanol and dilute to 100 μg / ml for use.
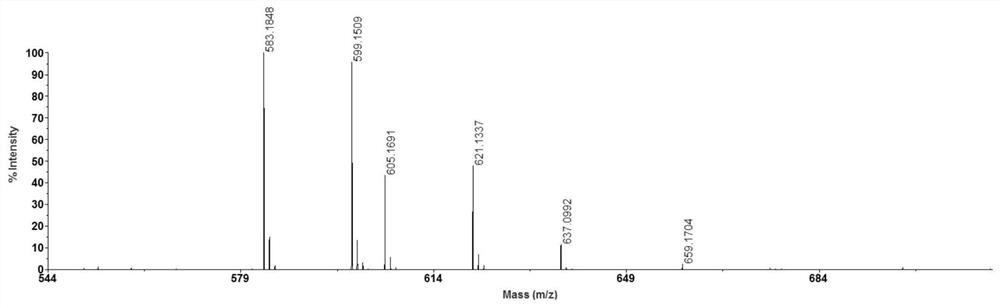
[0033] After mixing equal volumes of N-butyl-4-hydroxy-1,8-naphthalimide solution and 100 μg / ml Aβ35-40 solution, take 0.5 μL of the mixed solution on a clean MALDI target plate and place in air MALDI-MS analysis was carried out after the solvent was naturally volatilized, and the results are shown in the attached image 3 shown. As can be seen from the attached figure, [M+Na] + 、[M+K] + , [M+2Na-H] + , [M+K+Na-H] + 、[M+2K-H] + , [M+2K+Na-2H] + The peaks are obvious, and the resolution and ...
Embodiment 3
[0035] Weigh 1 mg of N-butyl-4-hydroxy-1,8-naphthalimide and dissolve it in 1 ml of a mixed solution of 50% acetonitrile and water containing 0.2% trifluoroacetic acid, sonicate for 5 minutes, and collect the overgrowth after centrifugation The membrane supernatant was used for later use. Dissolve 500 μg of Aβ35-42 in 500 μl of methanol and sonicate for 5 minutes to obtain a clear Aβ35-42 solution. Dissolve 10 μL of this solution in 90 μL of methanol and dilute to 100 μg / ml for use.
[0036] After mixing equal volumes of N-butyl-4-hydroxy-1,8-naphthalimide solution and 100 μg / ml Aβ35-42 solution, take 0.5 μL of the mixed solution on a clean MALDI target plate and place in air MALDI-MS analysis was carried out after the solvent was naturally volatilized, and the results are shown in the attached Figure 4 shown. As can be seen from the attached figure, [M+Na] + 、[M+K] + , [M+2Na-H] + The peaks are obvious, and the resolution and signal-to-noise ratio are high.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com