Cell culture
A cell and medium technology, applied in the field of cell culture, can solve the problem of underestimating the toxicity of test substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
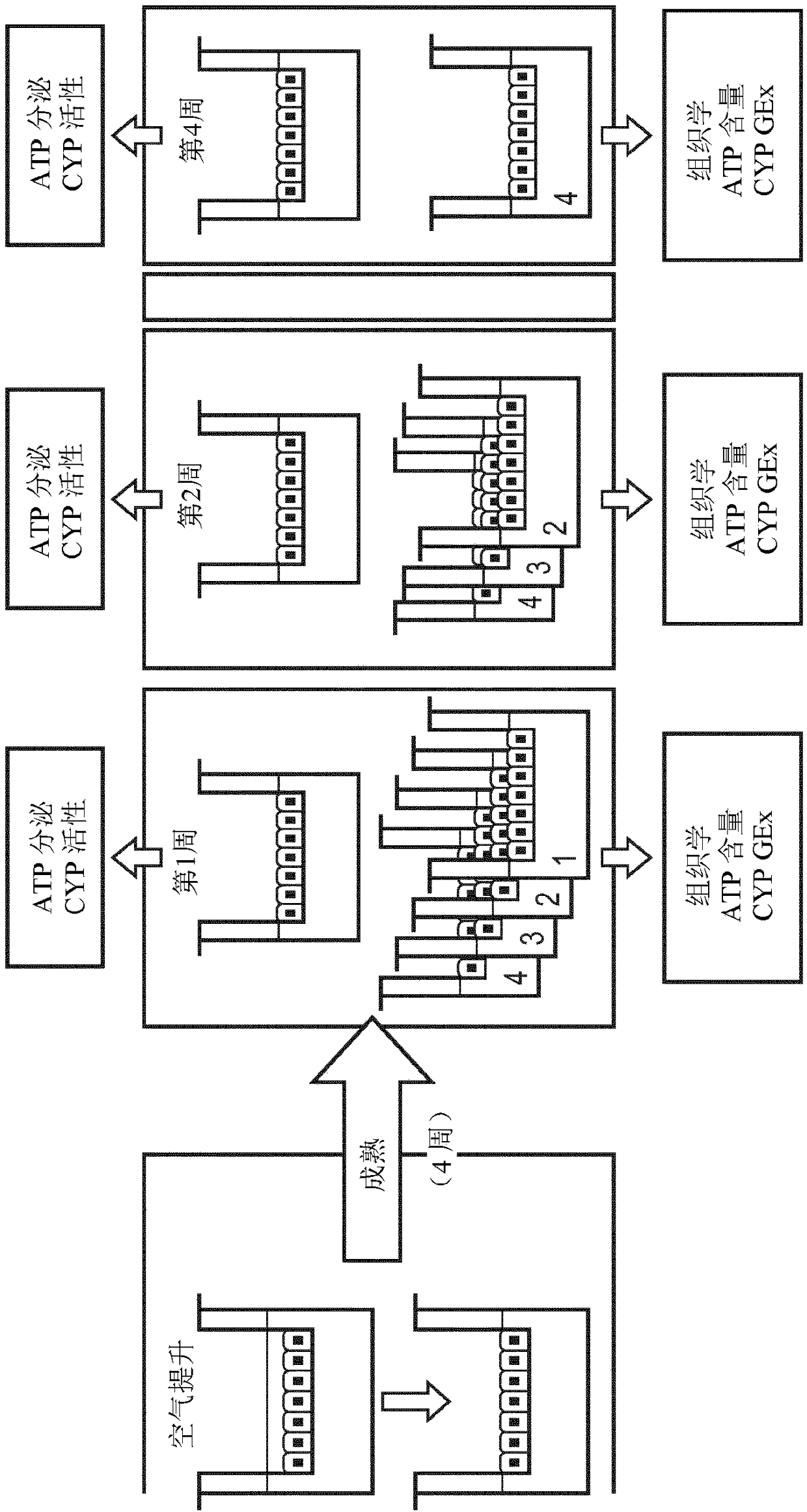
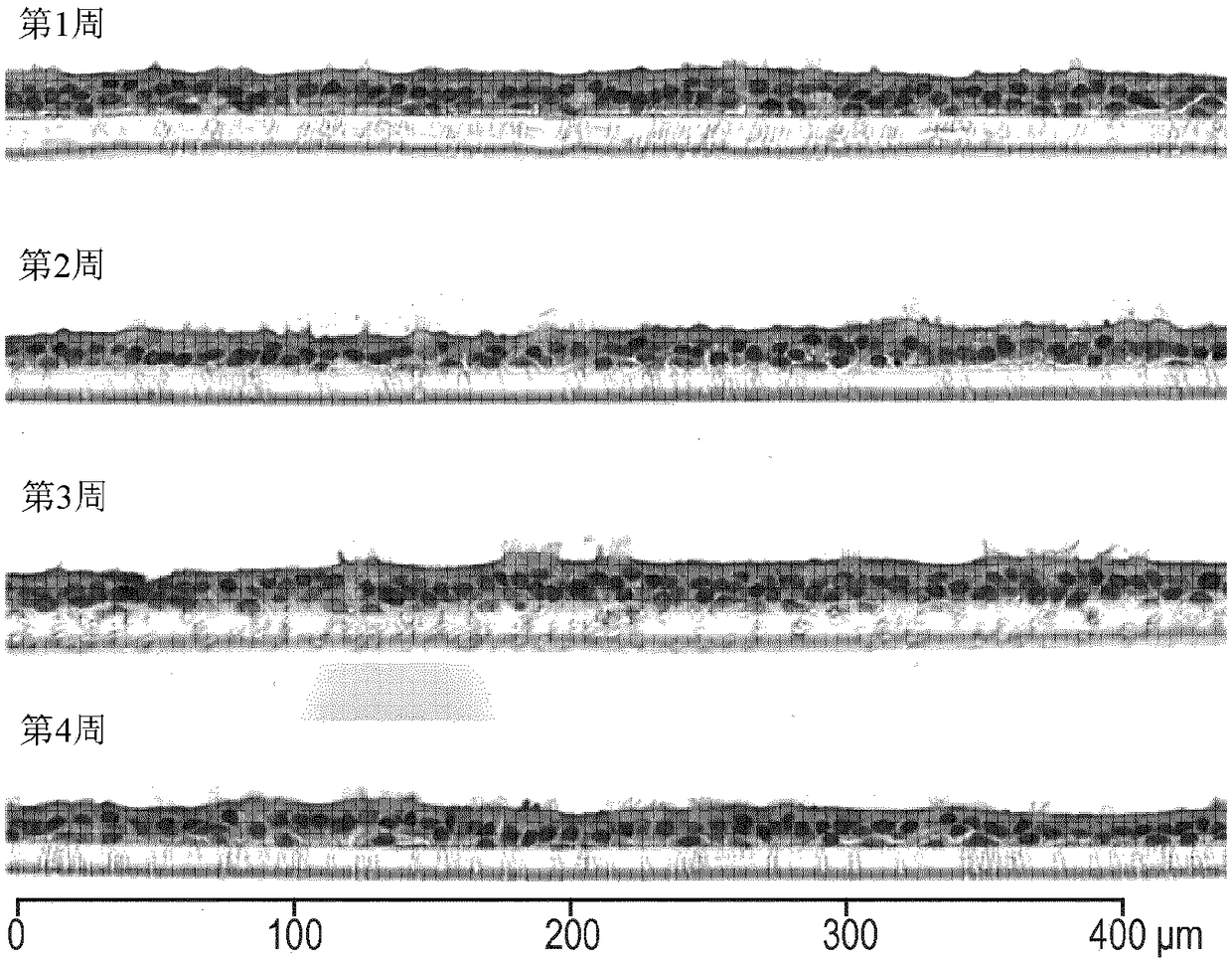
[0270] Preparation of bronchial organotypic cultures
[0271] These are based on a work titled Clonetics from Lonza (Basel, Switzerland) TM B-ALI TM Protocol for the preparation of air-liquid interface media. Briefly, normal human bronchial epithelial cells (NHBEC) were expanded in complete B-ALI medium at 37°C in 5% CO2 (90% relative humidity) until approximately 80% confluent. Cells were trypsinized, washed and resuspended. 35,000 cells were seeded onto type I collagen-coated insert ( root Switzerland) and the inserts were placed in multiwell plates prefilled with complete B-ALI medium (Lonza) and incubated for 3 days. The top medium was then removed and the basal medium was replaced with complete B-ALI medium. The air-lifted inserts were returned to the incubator, and the media was replaced every 2 to 3 days. In addition, a top wash is performed once a week during the ripening period. Cultures were used at maturity, usually 4 weeks after air lifting. Morphologic...
example 2
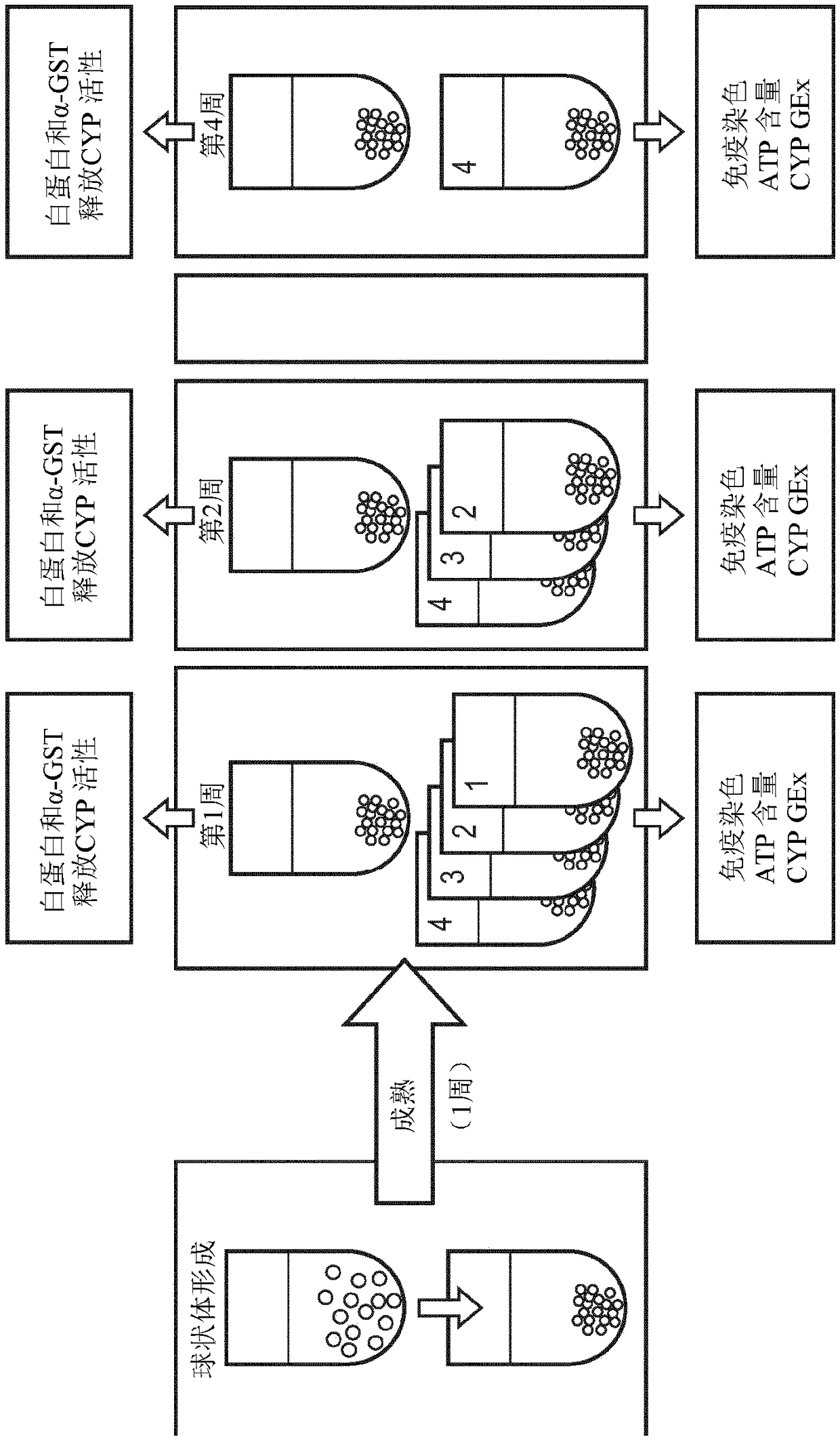
[0273] Preparation of liver spheroids
[0274] such as GravityTRAP from InSphero TM Prepare liver spheroids as described in the ULA plate manual. Briefly, HepaRG cells were first thawed at 37°C for 2 minutes and then mixed into 9 ml of pre-warmed William's E medium (ThermoFisher Scientific, reference 12551032) supplemented with thawed, coated and Universal Supplement (ThermoFisher Scientific, ref. HPRG770) and GlutaMAX solution (ThermoFisher Scientific, ref. 35050061). Cells were then centrifuged at 400 xg for 2 minutes, and the medium was replaced with fresh William's E medium with the same supplements as above. then Each well of a spheroid microplate (ref. 4520) distributes approximately 5000 cells. After 5 days, the medium was replaced by fresh William's E medium (ThermoFisher Scientific, ref. 12551032) supplemented with HepaRG maintenance and metabolism supplement (ThermoFisher Scientific, ref. HPRG720) and GlutaMAX solution (ThermoFisher Scientific, ref. 35050061). ...
example 3
[0276] Determination of Morphology of Bronchial Organotypic Cultures and Hepatic Spheroids
[0277] Morphology of bronchial organotypic cultures assessed after fixation and paraffin embedding, sectioning, and staining with hematoxylin and eosin (H&E) and alcian blue, as previously described in Toxicol Sci. 2015 9 Jan;147(1):207-21.
[0278] Hepatic spheroid morphology was assessed after immunostaining. Briefly, liver spheroids were fixed overnight in 4% fresh paraformaldehyde. After blocking in 1% Triton X-100 / 0.2% fish skin gelatin (FSG), the spheroids were treated with mouse anti-cytokeratin 19 (1 / 500, Abcam, Cambridge) diluted in PBS with 0.1% FSG. , UK) stained for 24 hours. Primary antibodies were visualized using FITC-labeled goat anti-mouse antibody (1 / 500, Abcam). Spheroids were then mounted using ProLong Diamond Antifade Agent (Thermo Fisher) with DAPI and evaluated by high content imaging on the Cellinsight™ CX7 platform (Thermo Fisher).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com