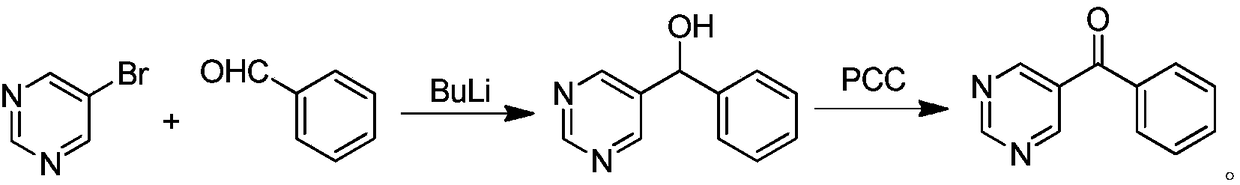
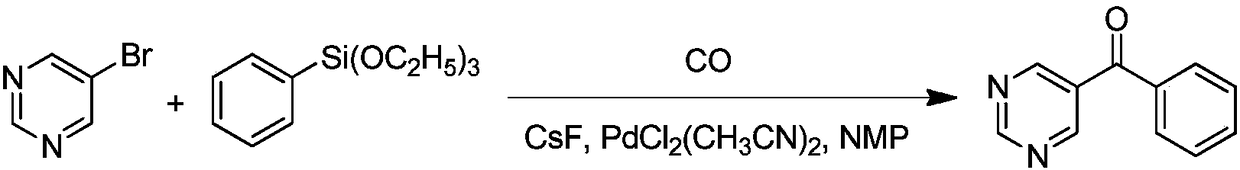
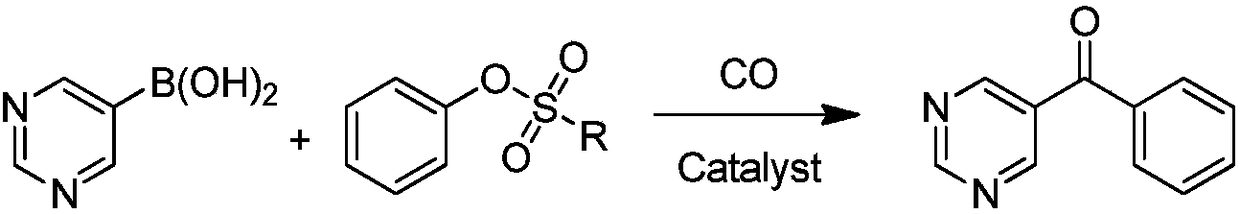
Synthetic method of OLED material intermediate benzoyl pyrimidine compound
A technology of benzoylpyrimidine and synthesis method, which is applied in the field of synthesis of benzoylpyrimidine compounds as intermediates of OLED materials, and can solve the problems of expensive phenylboronic acid and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] The synthesis of embodiment 1,5-benzoyl-2-hydroxypyrimidine
[0085] (1) In a 250ml three-necked flask, add 12g (0.174mol) of sodium ethoxide and 100mL of anhydrous toluene, stir, add dropwise (30 minutes) 20.5g (0.17mol) of acetophenone and 25.5g (0.174mol) of acetophenone The uniformly mixed solution of diethyl oxalate was heated to 50-60°C after dropping, and stirred for 4 hours. Cool to room temperature, wash with 5% dilute hydrochloric acid solution until the pH value of the eluent is 5-6, separate the liquids, collect the toluene layer (located on the upper layer), dry with anhydrous sodium sulfate for 30 minutes, filter, and the filtrate water pump reduces Toluene was recovered by pressure distillation, and the residue was recrystallized with 300ml of 95% ethanol to obtain 32.6g of pure ethyl ester intermediate I (purity: 99.0%), yield 87%, Mp.67-68°C.
[0086] (2) In a 25ml single-necked flask, add 2.8g (0.0125mol) of ethyl ester intermediate I, 4mL (0.025mol) ...
Embodiment 2
[0090] The synthesis of embodiment 2,5-benzoyl-2-hydroxypyrimidine
[0091] (1) In a 250ml three-necked flask, add 9.5g (0.174mol) of sodium methoxide and 100mL of dry xylene, stir, add dropwise (30 minutes) 20.5g (0.17mol) of acetophenone and 20.6g (0.174mol) of acetophenone ) a homogeneously mixed solution of dimethyl oxalate, the temperature was raised to 40-50° C. after dropping, and the reaction was stirred for 4 hours. Cool to room temperature, wash with 5% dilute hydrochloric acid solution, and adjust the pH value to 5-6, separate the layers, collect the xylene layer, dry it with anhydrous sodium sulfate for 30 minutes, filter, and recover the xylene by distilling the filtrate under reduced pressure with a water pump. The residue was recrystallized with 95% ethanol to obtain 30.2 g of the pure methyl ester intermediate I (purity: 98.9%), with a yield of 86%.
[0092] (2) In a 50ml single-necked flask, add 5.16g (0.025mol) of methyl ester intermediate I, 6mL (0.05mol) o...
Embodiment 3
[0096] The synthesis of embodiment 3,5-benzoyl-2-hydroxypyrimidine
[0097] (1) In a 250ml three-necked flask, add 25.5g (0.265mol) of sodium tert-butoxide and 100mL of dry cyclohexane, stir, add dropwise (30 minutes) 20.5g (0.17mol) of acetophenone and 51.0g (0.348mol) a homogeneously mixed solution of diethyl oxalate, the temperature was raised to 70-80°C after dropping, and the reaction was stirred for 4 hours. Cool to room temperature, wash with 5% dilute hydrochloric acid solution, and adjust the pH value to 5-6, separate the liquid, collect the cyclohexane layer, dry with anhydrous sodium sulfate for 30 minutes, filter, and the filtrate is distilled under reduced pressure by a water pump to recover the cyclohexane alkane, and the residue was recrystallized with 95% ethanol to obtain 30.8 g of the pure ethyl ester intermediate I (purity: 98.7%), with a yield of 82%.
[0098] (2) In a 50ml single-necked flask, add 5.6g (0.025mol) of ethyl ester intermediate I, 24mL (0.15m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com