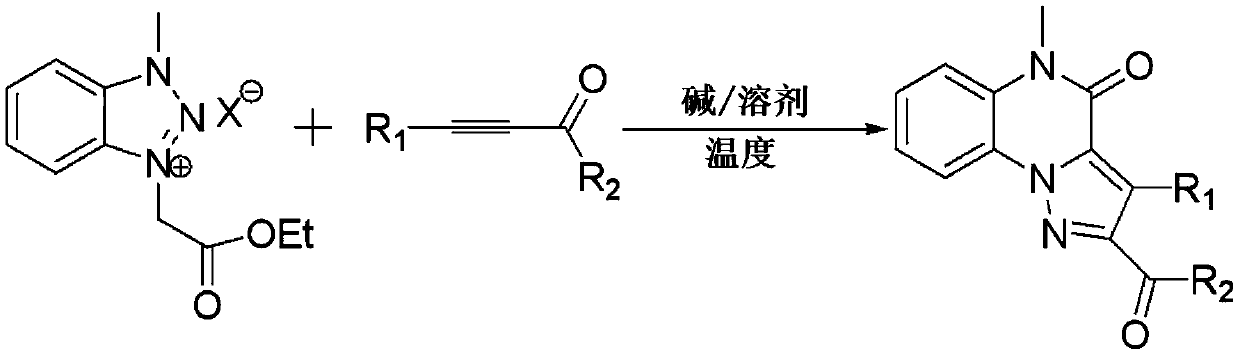
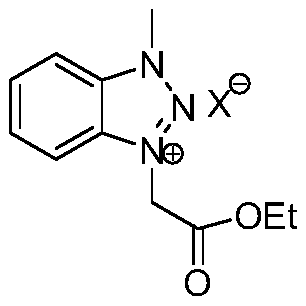
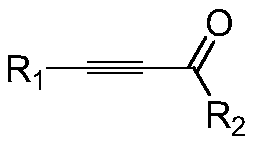
A kind of method for preparing pyrazole quinoxalinone derivative
A technology for pyrazole quinoxalinone and derivatives, which is applied in the field of preparation of pyrazole quinoxalinone derivatives, can solve the problems of high price, high toxicity, complex operation process, etc., and achieve rapid reaction, easy separation, and low raw material price cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of 2-benzoyl-5-methylpyrazol[1,5-a]quinoxalin-4(5H)-one
[0023]
[0024] At room temperature, add 0.30g (1mmol) 1-methyl-3-(ethoxycarbonylmethylene) benzotriazole ylide bromide, 0.130g (1mmol) acetylenone to a 50-ml round-bottomed flask, 20 mL of acetonitrile (CH3CN), stirred for 10 minutes, added 0.11 g (1 mmol) of potassium tert-butoxide (t-BuOK), followed by thin-layer chromatography. After the reaction was over, CH CN was recovered by concentration under reduced pressure, 10 ml of water and 20 ml of ethyl acetate were added to the residue, the layers were separated, the organic phase was extracted with 2×20 ml of ethyl acetate, the organic layers were combined, and then 2×20 ml Washed with saturated brine and dried over anhydrous sodium sulfate. Ethyl acetate was recovered by concentration under reduced pressure, and the residue was quickly separated by column chromatography to obtain 0.212 g of a light yellow solid product with a yield of 70%. mp....
Embodiment 2
[0026] Preparation of 2-(4-chlorobenzoyl)-5-methylpyrazol[1,5-a]quinoxalin-4(5H)-one
[0027]
[0028] At room temperature, add 0.30g (1mmol) 1-methyl-3-(ethoxycarbonylmethylene) benzotriazole ylide bromide, 0.165g (1mmol) 1-( 4-Chlorophenyl) prop-2-yn-1-one, 20mL N,N-dimethylformamide (DMF), after stirring for 10 minutes, add 0.138g (1mmol) potassium carbonate (K2CO3), use TLC Chromatography trace. After the reaction, concentrate under reduced pressure to recover DMF, add 10 ml of water and 20 ml of ethyl acetate to the residue, separate the layers, extract the organic phase with 2 × 20 ml of ethyl acetate, combine the organic layers, and then use 2 × 20 ml Washed with saturated brine and dried over anhydrous sodium sulfate. Ethyl acetate was recovered by concentration under reduced pressure, and the residue was quickly separated by column chromatography to obtain 0.324 g of a light yellow solid product with a yield of 96%. mp.227-230℃; 1H NMR (600MHz, CDCl3) δ: 8.41 (d...
Embodiment 3
[0030] Preparation of 2-(4-fluorobenzoyl)-5-methylpyrazol[1,5-a]quinoxalin-4(5H)-one
[0031]
[0032]At room temperature, add 0.3g (1mmol) 1-methyl-3-(ethoxycarbonylmethylene) benzotriazole ylide bromide, 0.148g (1mmol) 1-( 4-Fluorophenyl) prop-2-yn-1-one, 20mL N,N-dimethylformamide (DMF), after stirring for 10 minutes, add 0.1382g (1mmol) potassium carbonate (K2CO3), use TLC Chromatography trace. After the reaction, concentrate under reduced pressure to recover DMF, add 10 ml of water and 20 ml of ethyl acetate to the residue, separate the layers, extract the organic phase with 2 × 20 ml of ethyl acetate, combine the organic layers, and then use 2 × 20 ml Washed with saturated brine and dried over anhydrous sodium sulfate. Ethyl acetate was recovered by concentration under reduced pressure, and the residue was quickly separated by column chromatography to obtain 0.315 g of a light yellow solid product with a yield of 98%. mp.140-143℃; 1H NMR (600MHz, CDCl3) δ: 8.49–8.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com