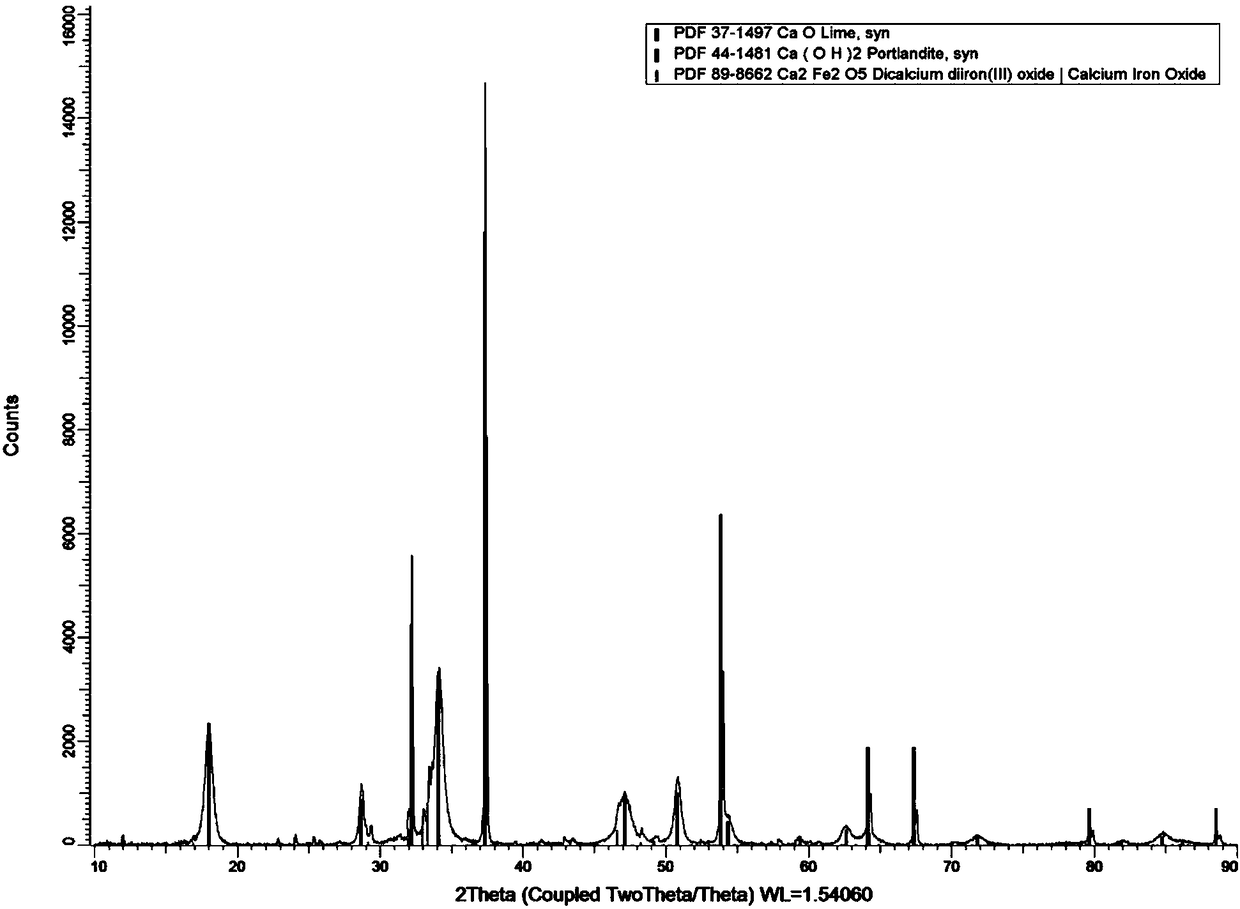
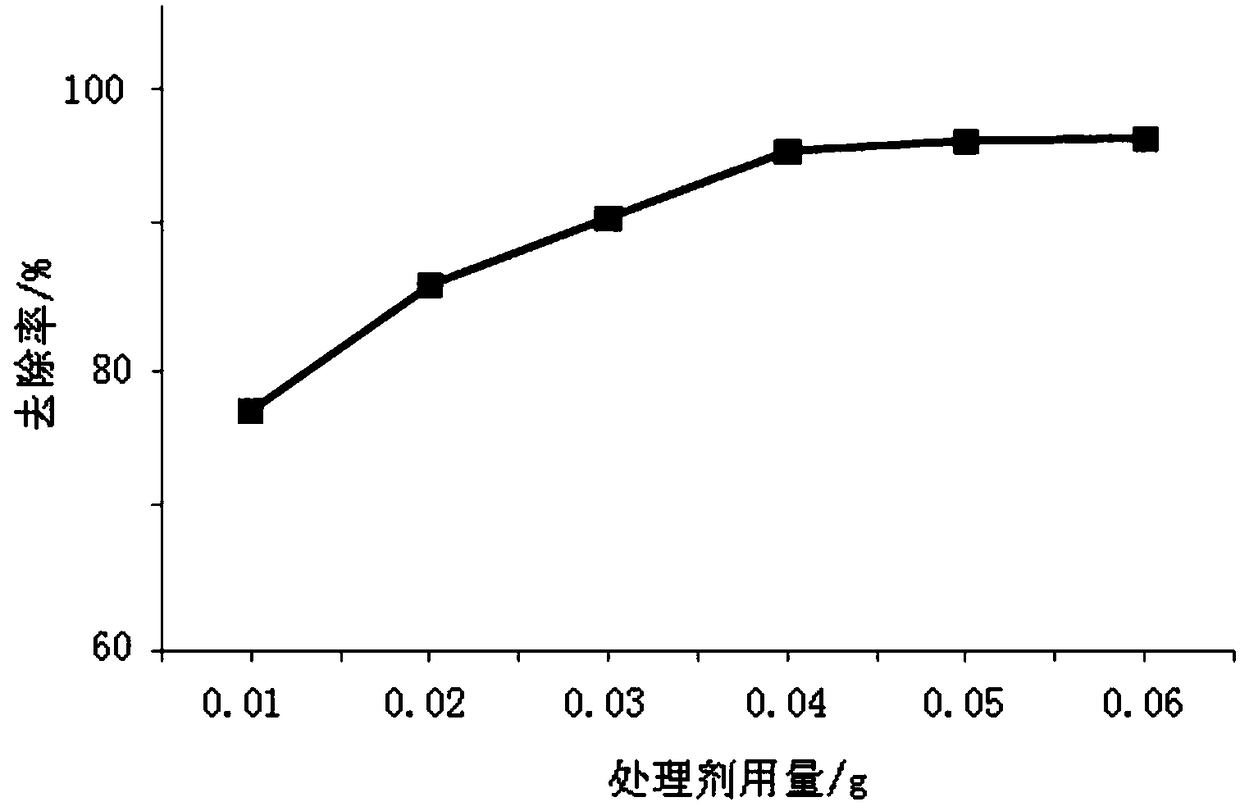
A preparing method and application of an oyster shell powder-ferric oxide nanometer composite material
An iron oxide nanometer and composite material technology is applied in the field of preparation of oyster shell powder iron oxide nanocomposite materials, which can solve the problems of low added value of oyster shells, pollution of oyster shells, weak adsorption capacity and the like, and achieves a simple preparation method and good adsorption. Removal power, easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Preparation of nanocomposite precursor powder:
[0023] Soak 100-mesh oyster shell powder in 0.15 mol·L -1 The ferric nitrate solution (the solid-to-liquid ratio of powder to solution (g:mL) is 1:4) was stirred and soaked at room temperature for 12 h, filtered and dried to obtain the nanocomposite precursor powder.
[0024] 2. Preparation of composite materials:
[0025] Put the above-mentioned nanocomposite precursor powder into a crucible and place it in a high-temperature furnace, heat it up to 900°C at a rate of 300°C / h and keep it warm for 6 hours for calcination reaction. After the reaction is completed, naturally cool to room temperature and take it out to obtain oyster shells Powdered iron oxide nanocomposites.
Embodiment 2
[0027] 1. Preparation of nanocomposite precursor powder:
[0028] Soak 100-mesh oyster shell powder in 0.10 mol·L -1 In ferric nitrate solution (the ratio of powder to solution (g:mL) is 1:4), stir and soak at room temperature for 12 h, filter and dry to obtain the nanocomposite precursor powder.
[0029] Second, the preparation of nanocomposites:
[0030] Put the above-mentioned nanocomposite precursor powder into a crucible and place it in a high-temperature furnace, heat it up to 900°C at a rate of 300°C / h and keep it warm for 6 hours for calcination reaction. After the reaction is completed, naturally cool to room temperature and take it out to obtain oyster shells Powdered iron oxide nanocomposites.
Embodiment 3
[0032] 1. Preparation of rice composite material precursor powder:
[0033] Soak 100-mesh oyster shell powder in 0.05 mol·L -1 In ferric nitrate solution (the ratio of powder to solution (g:mL) is 1:4), stir and soak at room temperature for 12 h, filter and dry to obtain the nanocomposite precursor powder.
[0034] Second, the preparation of rice composite materials:
[0035] Put the above-mentioned nanocomposite precursor powder into a crucible and place it in a high-temperature furnace, heat it up to 900°C at a rate of 300°C / h and keep it warm for 6 hours for calcination reaction. After the reaction is completed, naturally cool to room temperature and take it out to obtain oyster shell powder Iron oxide nanocomposites.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com