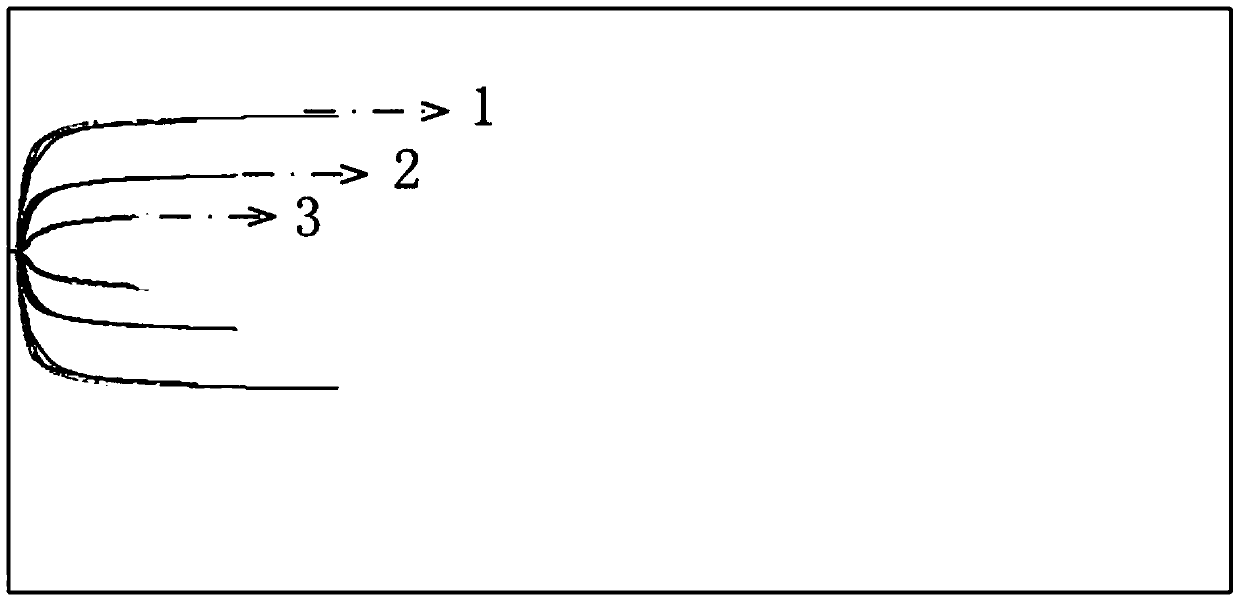
A thrombosis elastic diagram instrument control product and its preparation methods and applications
A technology of thromboelastography and quality control products, which is applied in the direction of material inspection products and biological tests, can solve the problems of poor accuracy and stability, and achieve the effects of accurate value determination, high discrimination and good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
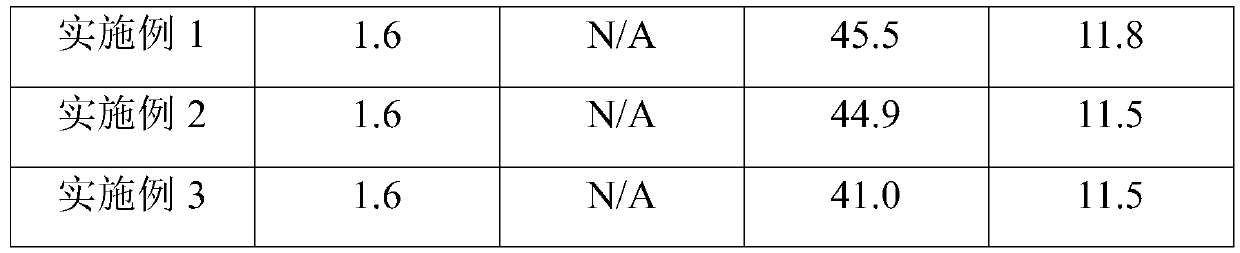
Embodiment 1
[0053] The invention provides a quality control product for a thromboelastography instrument, including a quality control product L1 and a quality control product L2;
[0054] Quality Control L1 includes raw materials in the following volume ratios:
[0055] Serum matrix solution 490, fibrinogen matrix solution 210, tissue factor 1.5, coagulation activator kaolin aqueous solution 10;
[0056] Quality Control L2 includes raw materials in the following volume ratios:
[0057] Serum matrix solution 650, fibrinogen matrix solution 50, tissue factor 1.5, coagulation activator kaolin aqueous solution 10;
[0058] Among them, the serum matrix solution is a mixed solution containing 8% of plasticizer mannitol and 5% of bovine serum albumin; solution; the mass concentration of kaolin aqueous solution is 1.0mg / mL; in the quality control product L1, the mass fraction of sodium citrate contained is 3.8‰, and the platelet content is 121×10 9 pc / L; in the quality control product L2, the ...
Embodiment 2
[0072] The invention provides a quality control product for a thromboelastography instrument, including a quality control product L1 and a quality control product L2;
[0073] Quality Control L1 includes raw materials in the following volume ratios:
[0074] Serum matrix solution 580, fibrinogen matrix solution 120, tissue factor 1.0, coagulation activator kaolin aqueous solution 6;
[0075] Quality Control L2 includes raw materials in the following volume ratios:
[0076] Serum matrix solution 620, fibrinogen matrix solution 80, tissue factor 2.0, coagulation activator kaolin aqueous solution 8;
[0077] Among them, the serum matrix solution is a mixed solution containing 8% plasticizer mannitol and 5% bovine serum albumin; the fibrinogen matrix solution is a mixed solution of fibrinogen and platelets; In the control product L1, the mass fraction of sodium citrate contained was 3.8‰, and the platelet content was 97×10 9 pc / L; in the quality control product L2, the mass fra...
Embodiment 3
[0091] The invention provides a quality control product for a thromboelastography instrument, including a quality control product L1 and a quality control product L2;
[0092] Quality Control L1 includes raw materials in the following volume ratios:
[0093] Serum matrix solution 400, fibrinogen matrix solution 300, tissue factor 2.0, coagulation activator kaolin aqueous solution 8;
[0094] Quality Control L2 includes raw materials in the following volume ratios:
[0095]Serum matrix solution 680, fibrinogen matrix solution 20, tissue factor 2.0, coagulation activator kaolin aqueous solution 6;
[0096] Wherein, the serum matrix solution is a mixed solution containing plasticizer glycine 5.6)% and sucrose 4.8%; the fibrinogen matrix solution is a fibrinogen saturated solution formed by dissolving fibrinogen and platelets in a Tris buffer at pH=7.6; The mass concentration of kaolin aqueous solution is 1.0 mg / mL; in the quality control product L1, the mass fraction of sodium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com