Preparation method for photo-Fenton catalyst
A catalyst, Fenton's technology, applied in the field of photo-Fenton catalyst preparation, can solve the problems of restricting development and application, difficult to treat sludge, etc., and achieve the effect of enhancing adsorption and promoting the separation of photogenerated charges
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation method of present embodiment composite catalyst is as follows:
[0028] Preparation of α-Fe 2 o 3 : First weigh 1.35g FeCl 3 ·6H 2 O. Mix 1 g of polyethylene glycol-20000 and 3.6 g of sodium acetate into 40 ml of ethylene glycol, and stir for 240 min with a magnetic stirrer to fully dissolve the polyethylene glycol-20000. Add the solution to a 50ml reaction kettle, then place the reaction kettle in a blast drying oven, keep it warm at 200°C for 8 hours, centrifuge and wash after the reaction to obtain Fe 3 o 4 powder. the Fe 3 o 4 The powder was added to an alumina crucible and calcined at 550°C for 2 hours to obtain α-Fe 2 o 3 , denoted as F.
[0029] Preparation of α-Fe 2 o 3 @C and surface treatment: Weigh 0.2gα-Fe respectively 2 o 3 and 2.0 g of glucose were added to 40 ml of distilled water, and ultrasonically mixed for 30 min to make them evenly mixed. The suspension was transferred to a 50ml reactor, and placed in a dry oven at 180°...
Embodiment 2
[0032] The difference between embodiment 2 and embodiment 1 is: in the preparation of α-Fe 2 o 3 When @C, the addition of glucose is 1.0g, other conditions are all the same, the α-Fe prepared by embodiment 2 2 o 3 @C@Bi 2 o 3 -Bi 2 WO 6 The catalyst is noted as FCB1.
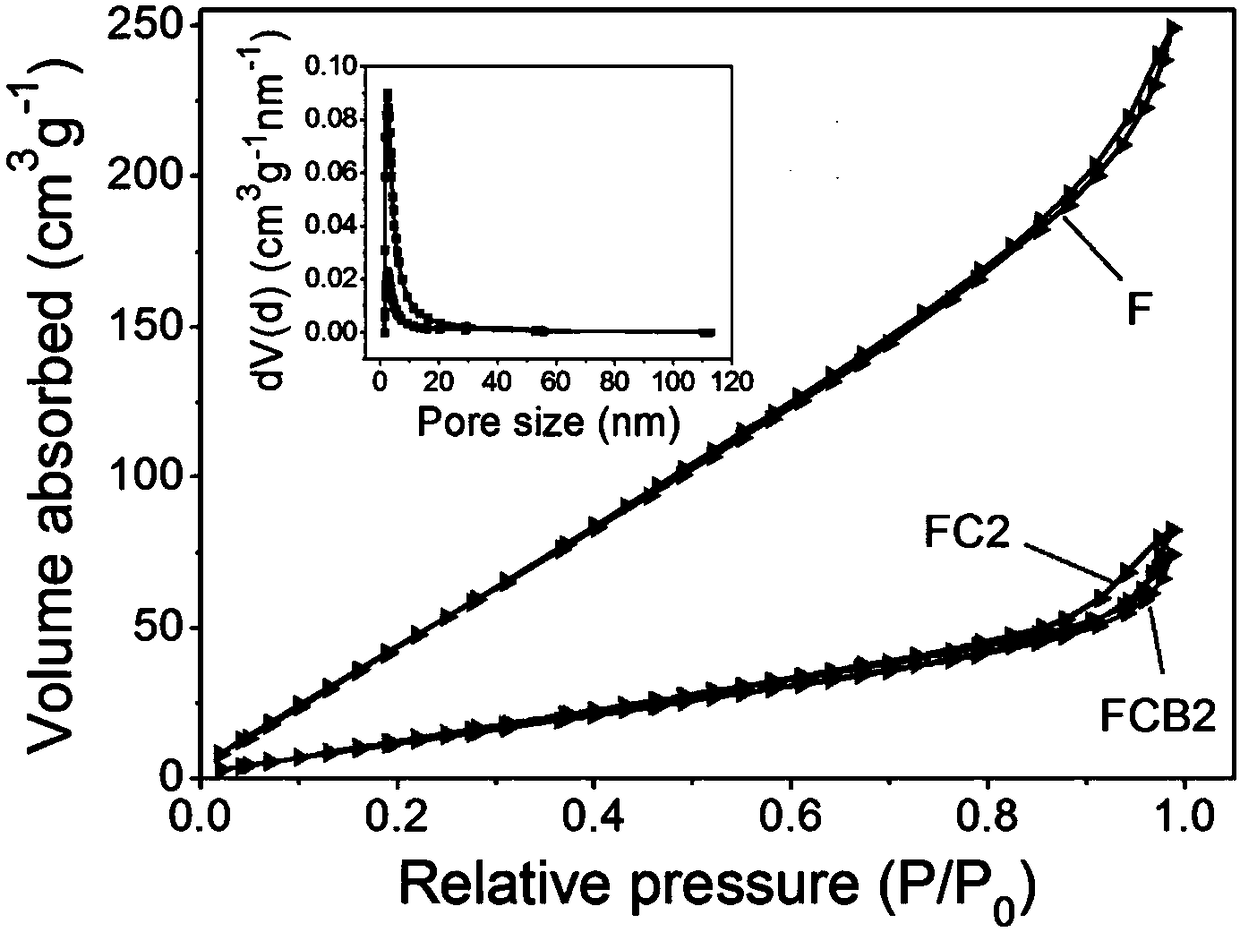
[0033] Result representation
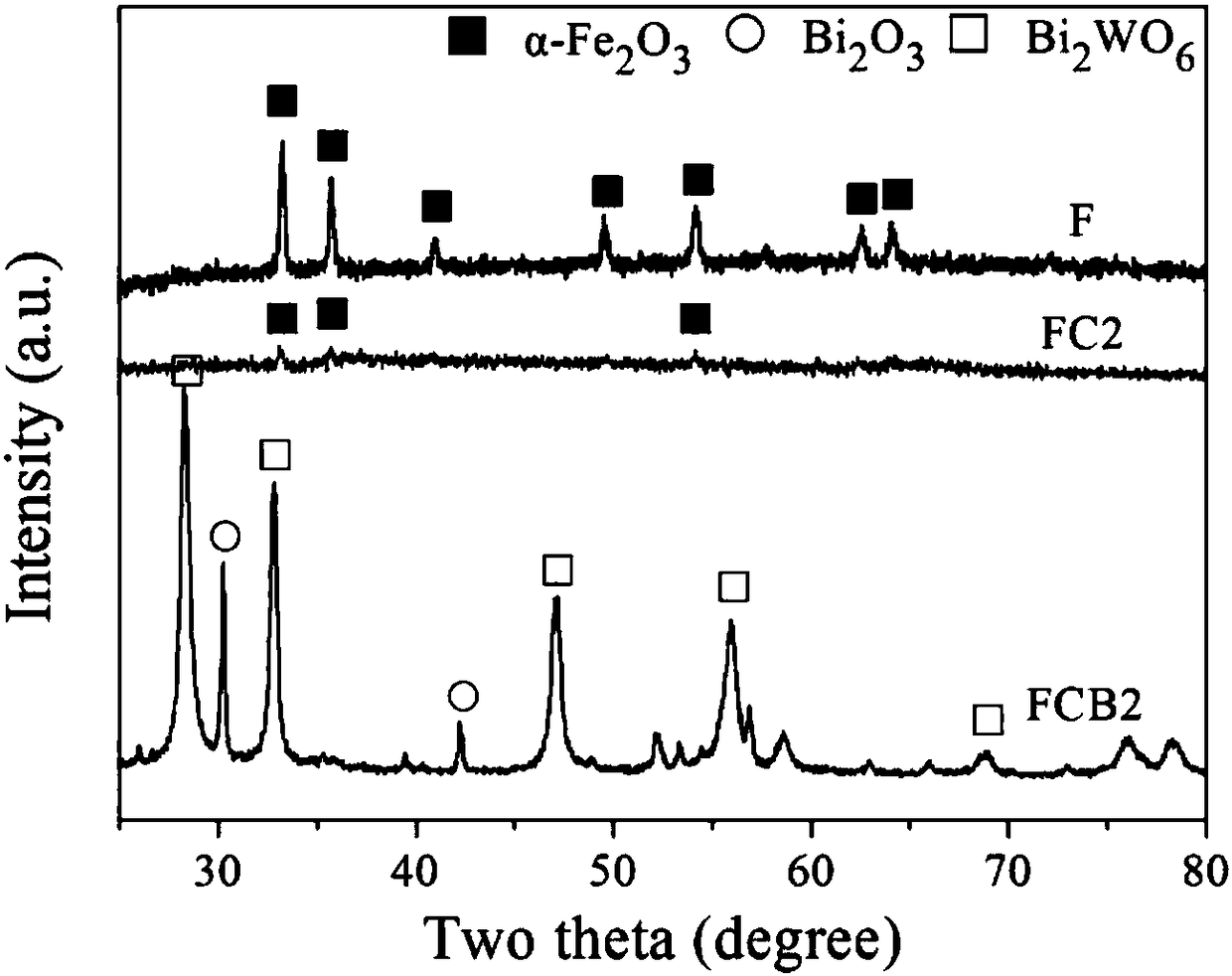
[0034] figure 1 α-Fe prepared for Example 1 2 o 3 , α-Fe 2 o 3 @C and α-Fe 2 o 3 @C@Bi 2 o 3 -Bi 2 WO 6 The X-ray diffraction pattern, in the figure F, FC2 and FCB2 correspond to the multi-step hydrothermal (solvothermal) method to prepare α-Fe 2 o 3 , α-Fe 2 o 3 @C and α-Fe 2 o 3 @C@Bi 2 o 3 -Bi 2 WO 6 .
[0035] There are obvious diffraction peaks in the F spectrum at 33.24°, 35.69°, 41.02°, 49.54°, 54.18°, 62.54° and 64.07°, which correspond to α-Fe 2 o 3 The (104), (110), (113), (024), (116), (214) and (300) crystal planes match JCPDS card No.87-1166. The diffraction peaks of the FC2 spectrum become weaker, and there are only three weak peaks at 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com