Alkaline xylanase as well as coding gene and application thereof
A xylanase and alkaline technology, applied in the field of alkaline xylanase and its coding gene and application, can solve the problems of high cost, poor direct utilization effect, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
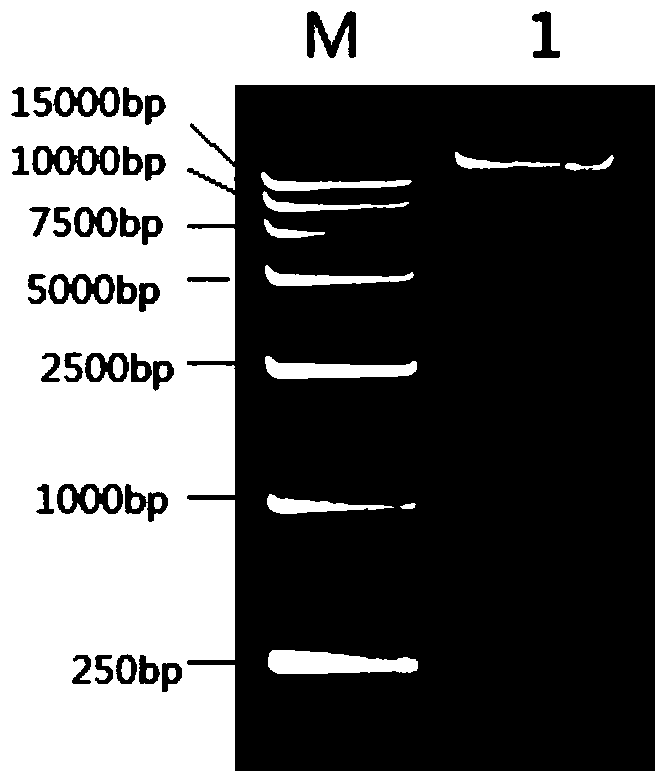
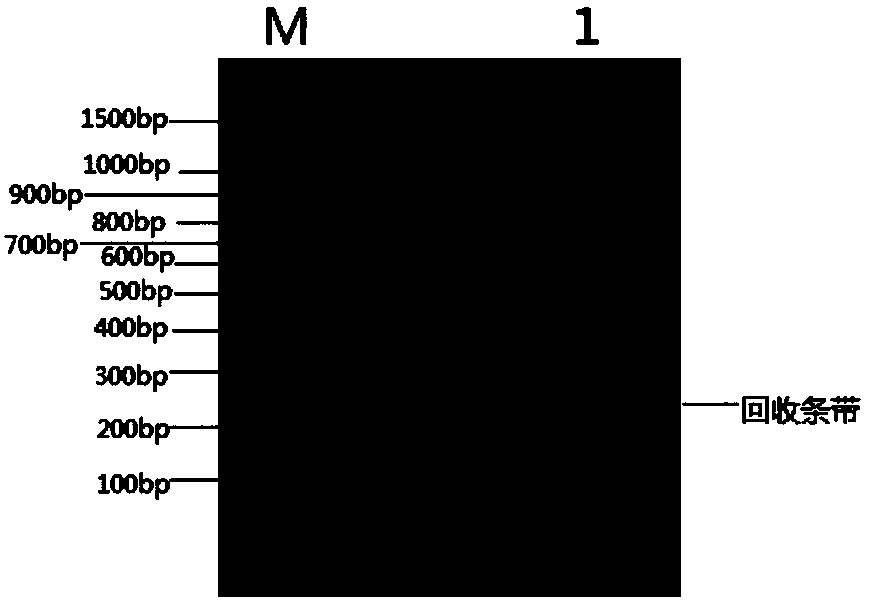
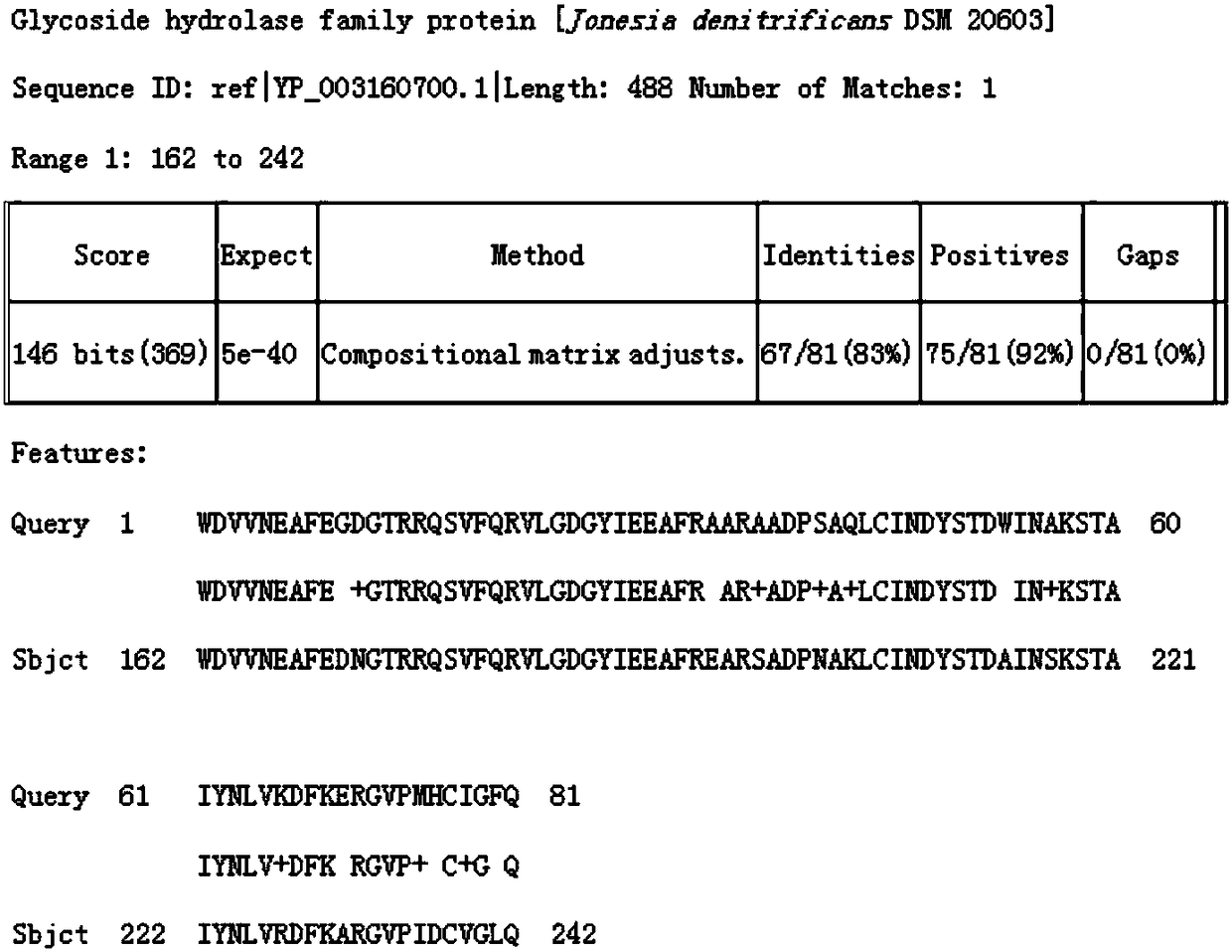
Image
Examples
Embodiment 1
[0053] (1) Materials
[0054] 1. Bacterial species: Streptomyces alkalophilus, which is an alkaline xylanase-producing strain Streptomyces sp.WMN-3 screened from a soil sample on a certain coast in Shenzhen. CCTCC), deposit time: September 7, 2017, deposit address: China. Wuhan. Wuhan University, deposit number: CCTCC NO: M 2017477. E.coli TOP10F' was purchased from Invitrogen;
[0055] 2. Vector: The vector used for DNA connection is pMD-19T Simple Vector produced by TaKaRa Company.
[0056] 3. Medium
[0057] (1) Enrichment medium: xylan 8.0g, peptone 10g, NaCl 15g, KH 2 PO 4 1.5g, Na 2 HPO 4 12H 2 O9.0g, MgSO 4 ·7H 2 O 2.0g, dissolved in 1L ddH 2 O, pH 9.0.
[0058] (2) Selection medium: xylan 8.0g, KNO 3 1.0g, MgSO 4 ·7H 2 O 0.5g, NaCl 15g, KH 2 PO 4 1.5g, 20g agar, dissolved in 1L ddH 2 O, pH 9.0.
[0059] (3) Slant medium (preserved strain): 1.0% glucose, 0.5% peptone, 0.5% yeast extract, 0.1% KH 2 PO 4 , 0.02% MgCl 2 , 5.0% NaCl, 1.0% Na 2 CO 3 ...
Embodiment 2
[0151] (1) Materials
[0152] 1. Strain: Streptomyces sp. WMN-3 strain (CCTCC NO: M 2017477). The host bacteria E.coli BL21 Star(DE3) and E.coli TOP10F' were purchased from Invitrogen;
[0153] 2. Vector: Escherichia coli expression vector pET-28a(+) (Novagen, Kan R ) was purchased from Novagen; vector map see Image 6 , its multiple cloning site map is shown in Figure 7 .
[0154] 3. Medium and buffer
[0155] (1) Selection medium: xylan 8.0g / L, KNO 3 1.0g / L, MgSO 4 ·7H 2 O 0.5g / L, NaCl 15g / L, KH 2 PO 4 1.5g / L, solid medium plus agar 15-20g / L, pH 9.0;
[0156] (2) LB medium: tryptone 10g / L, yeast extract 5g / L, NaCl 10g / L, solid medium plus agar 15-20g / L, pH 9.0, autoclaved at 121°C for 20min.
[0157] (3) TE buffer: 10mmol / L Tris-Hcl, pH8.0, 1mmol / L EDTA, pH8.0.
[0158] (4) Alkaline lysis solution I, II, III (plasmid extraction): Alkaline lysis solution I: glucose 50mmol / L, Tris-HCl (pH8.0) 25mmol / L, EDTA 10mmol / L. About 100mL per bottle, store at 4°C after s...
Embodiment 3
[0230] The enzymatic property research of embodiment 3 alkaline xylanase
[0231] (1) Experimental method
[0232] 1. Optimum reaction pH and pH stability
[0233] Take an appropriate amount of the pure enzyme solution of alkaline xylanase obtained in Example 2, add 1% xylan solutions with different pH values respectively, and measure the enzyme activity according to a conventional method. At the same time, the pure enzyme solution was stored at 37° C. for 60 min under different predetermined pH conditions, and then the remaining enzyme activity was measured by conventional methods.
[0234] 2. Optimum reaction temperature and temperature stability
[0235] Take an appropriate amount of the pure enzyme solution of alkaline xylanase obtained in Example 2, respectively place it under different predetermined temperature conditions and react for 20 minutes, and measure its enzyme activity. At the same time, the pure enzyme solution was placed under different predetermined tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com